Lung Ultrasound
Rationale.
THE QUESTION
Given the poor acoustic impedance of air, pairing ultrasound waves and an air-filled cavity with the expectation of obtaining diagnostic images might seem counterintuitive. However, the systematic study of sonographic patterns and artefacts allows the identification of reliable “profiles” to diagnose thoracic pathology [10].
The artefact-based approach to lung ultrasound is perhaps less straightforward than other POCUS modalities. Still, it can provide substantial information about the condition of the lungs and elucidate the presence of pleural fluid, pneumothorax, pulmonary oedema, or consolidation.
When performing lung POCUS, there are several questions to ask depending on the clinical picture. Still, the focus should remain within the binary model (yes/no) that we already know.
In the septic patient: Is there a consolidation?
In the cardiac patient: Is there pulmonary oedema or effusion?
In the shocked patient: Is there a tension pneumothorax?
In the trauma patient: Is there pneumothorax or haemothorax?
In the breathless patient: Is there pneumonia, pneumothorax, pleural effusion, pulmonary oedema, ARDS, COPD, or PE?
WHY USE ULTRASOUND?
In trauma, massive haemothorax and pneumothorax are common and rapidly fatal conditions if not detected and treated. Although the chest X-ray remains the initial investigation of choice for detecting these problems, in reality, it is not sensitive enough, and its reliability is often overestimated. For both pneumothorax and pleural effusion, chest X-ray sensitivity is around 50% (3, 4).
Additionally, in the ED setting, physical examination alone is neither reliable for the diagnosis nor able to accurately differentiate pulmonary oedema from pneumonia.
Lung US is non-invasive, quick and dynamic, allowing real-time monitoring at the bedside. It is more sensitive and reliable than a chest X-ray in finding pleural fluid, as it can detect as little as 100 mL of free fluid with 94% sensitivity and 98% specificity (4). In expert hands, lung US has been described as 98% sensitive and 99% specific for pneumothorax, and 85.7% sensitive and 98% specific for pulmonary oedema (1, 3, 5).
CONTRAINDICATIONS & LIMITATIONS
The only absolute contraindication to performing lung US is the presence of a more pressing problem (namely airway obstruction).
Lung US cannot determine the nature of pleural fluid: for example, fresh blood versus transudate. It cannot rule out alveolar consolidation or a tiny pneumothorax. Furthermore, the accuracy of the scan is very operator-dependent, and the inexperienced sonologist should be particularly wary of ruling out the conditions described. For a beginner, it might be wiser to use it as a rule-in tool.
Technique & Views.
PATIENT’S POSITION
Ideally, as much as possible of the chest should be scanned, including the front, sides and back, yet all of this is often impossible in the trauma or resuscitation setting. The operator will have to adapt the technique to the patient’s condition, but as a minimum, scan the upper and lower anterior chest wall and the basal posterolateral region.
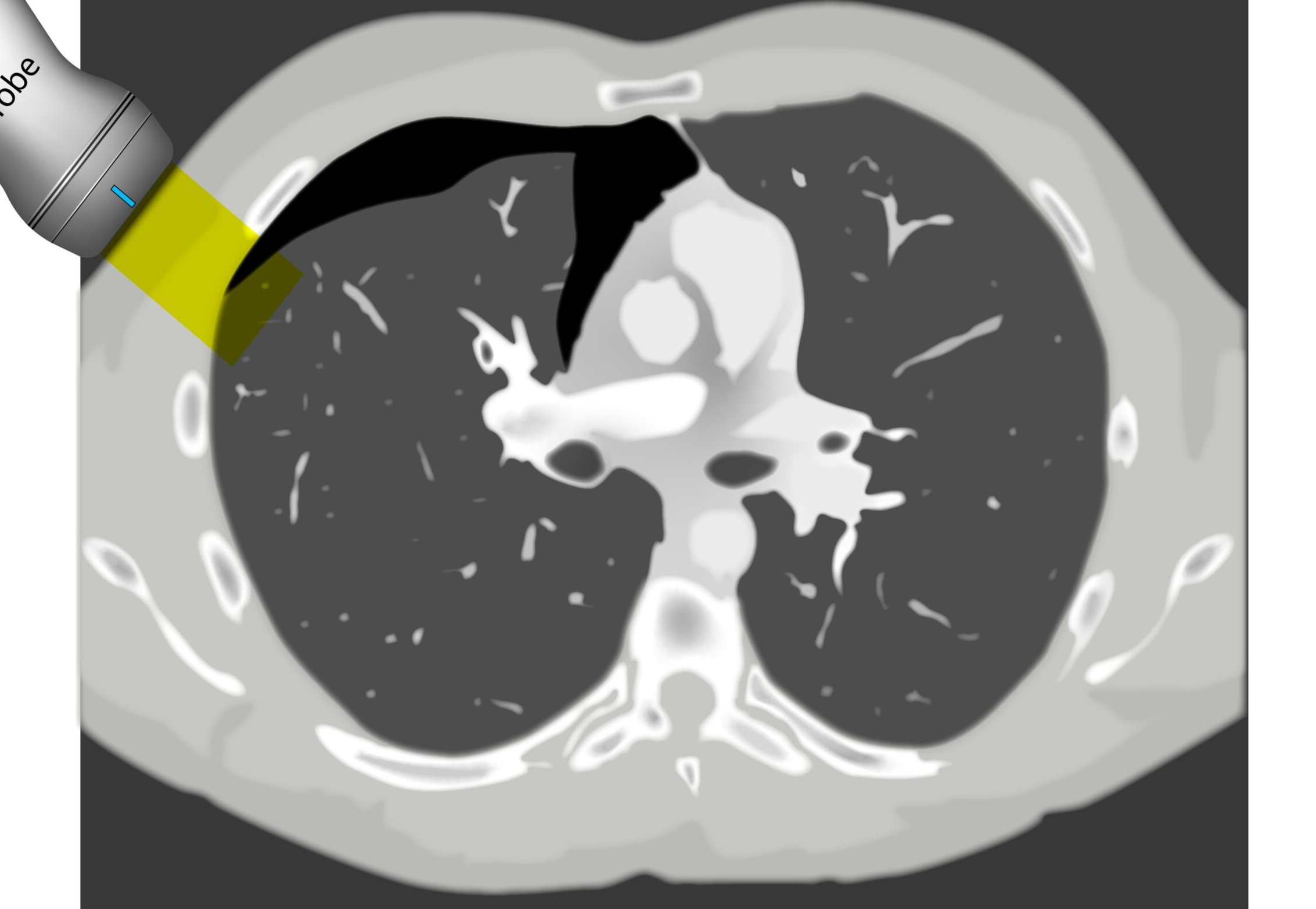
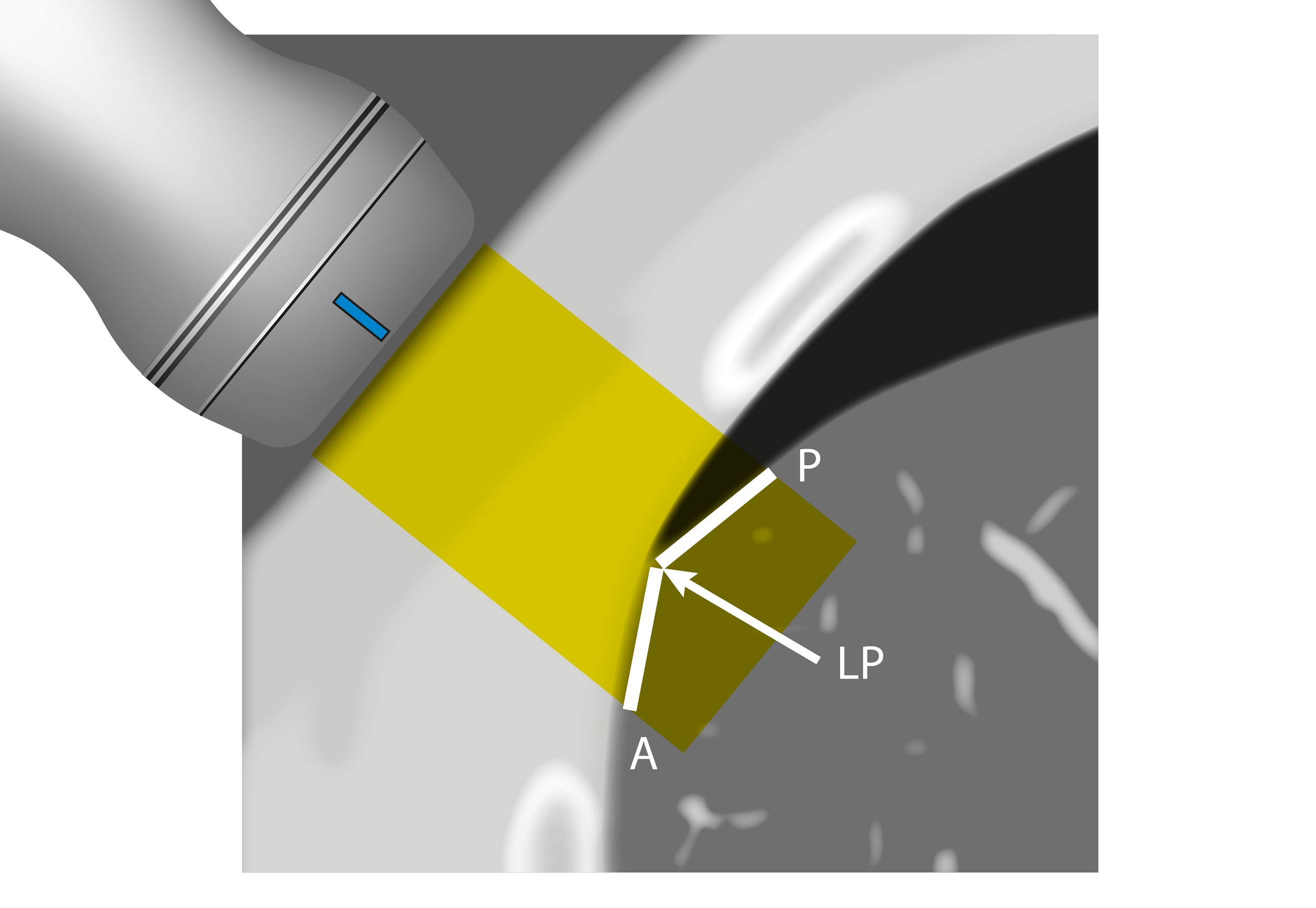
PROBE
The linear array probe is the most suitable to assess superficial structures such as the pleura. In comparison, the phased array probe has a low frequency and a small footprint which is designed to use the intercostal spaces as acoustic windows. If available, it provides the best overall view of the lung for studying conditions lying deeper to the pleura, namely the presence of fluid or consolidation. Still, the curved (abdominal) probe is an excellent option to start the study from the base, and it can be mastered to obtain good intercostal views of the lung.
THE VIEWS
There is no unique, standardised system to scan the lungs, and different protocols have been proposed, including from 3 to 20+ windows or quadrants. In general, the objective is to explore as much lung as possible and to do so in an orderly fashion to avoid missing significant pathology.
A simple systematic approach is to start at the lung base (as in a FAST scan). Set a deep depth and identify the diaphragm as a bright curved line. The “lung curtain” sign can be seen in a normal scan.
Then slide the probe up to the thorax while keeping it at a right angle to the ribs and scan along the mid and/or posterior axillary lines. The objective is to use the intercostal spaces as windows to visualize the pleural lines; accordingly, the depth and the gain should be adjusted for this purpose.
Ultimately, relocate to scan along the anterior chest wall (mid-clavicular line). This is fundamental to detect small pneumothoraces as trapped air will gather in the least dependent areas of the chest. In any area, identify the ribs by the shadows they cast and look between them in search of the highly echogenic pleural line.
Profiles: What to look for?
Ultrasound cannot directly visualise the normal, air-filled, pulmonary parenchyma. In the presence of air, however, the pleural layers are seen as highly reflective structures that normally slide against each other during the respiratory cycle.
Historically, lung ultrasound was supposed to be impossible (12). During the 90s, a forward-thinking French Intensivist, Daniel Lichtenstein, systematically identified several lung artefacts and correlated them with clinical aspects and other imaging modalities. This seminal and revolutionary work set the base for the current understanding of lung US, yet continues to grow in complexity and detail.
The fundamental concept of lung sliding, plus other artefacts and ultrasonographic signs have been grouped into distinct profiles that facilitate the diagnostic approach.
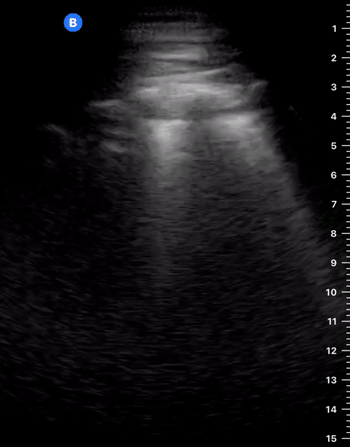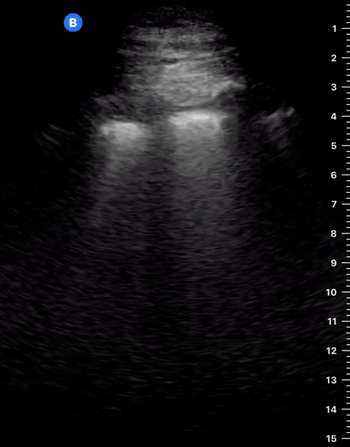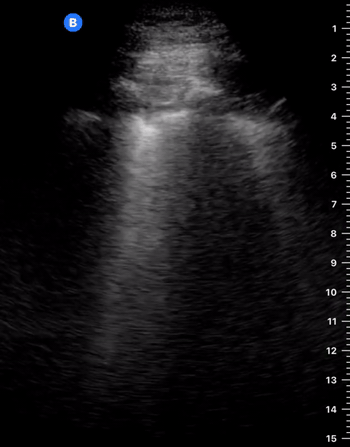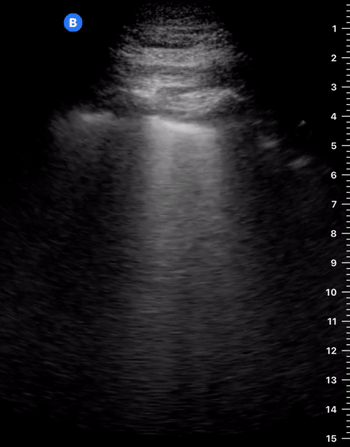
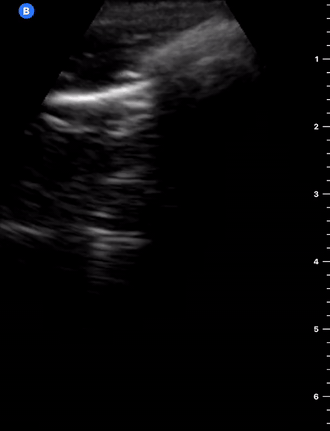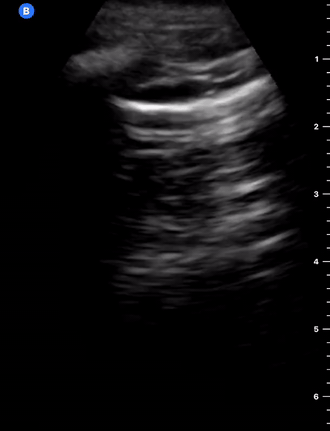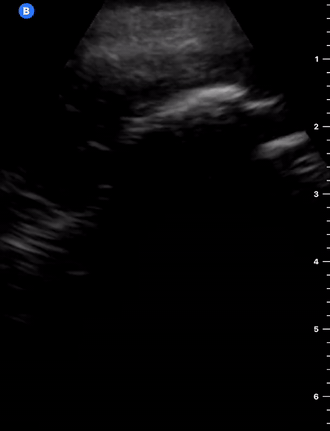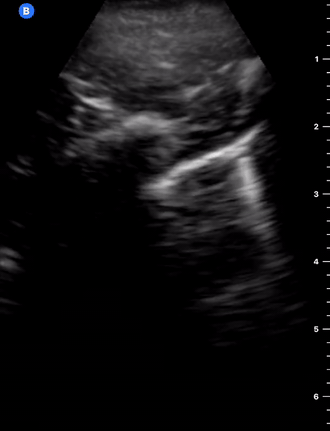
A-Profile
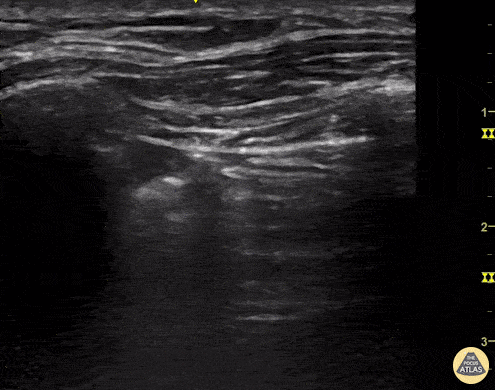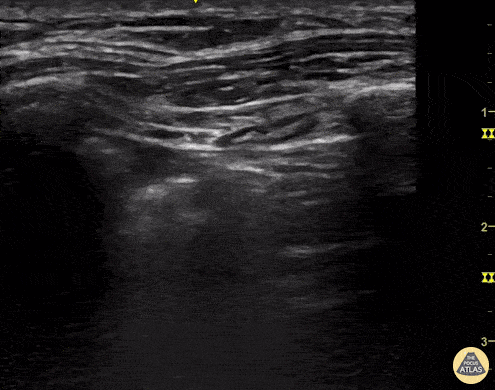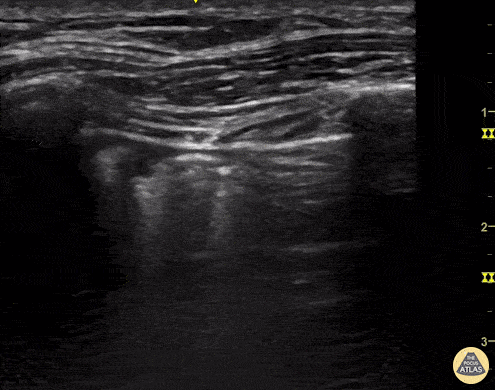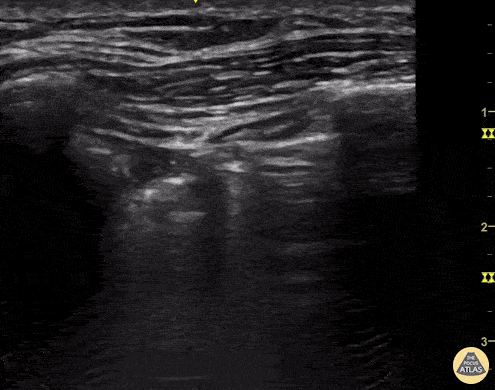
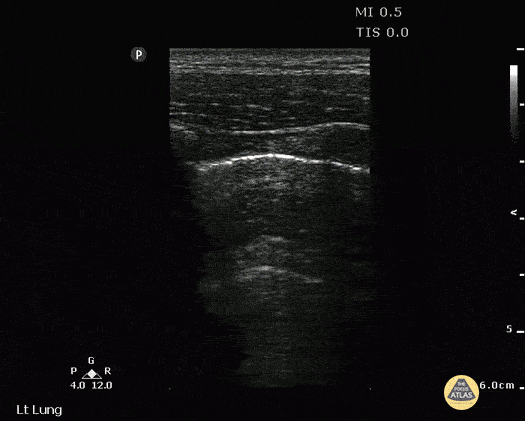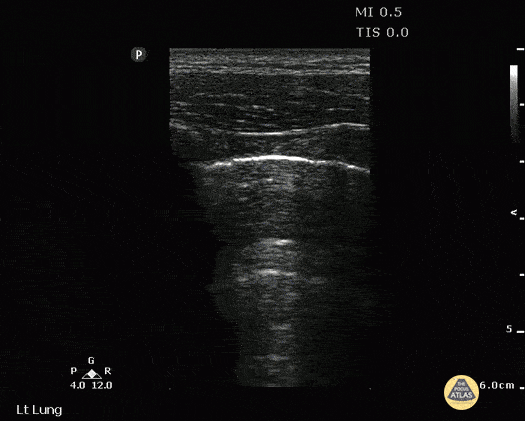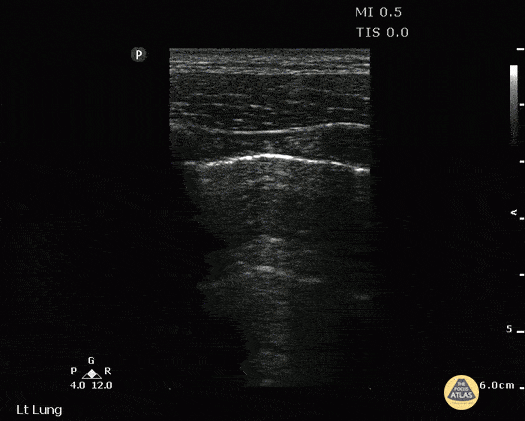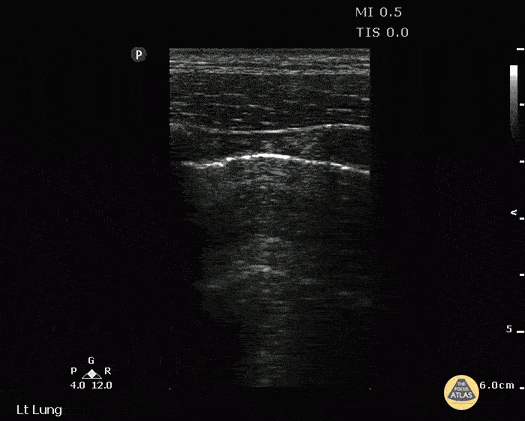
A-LINES & NORMAL LUNG
An A-profile is characterized by the presence of A-lines and dynamic lung sliding. Although it is called normal, this pattern can be found in patients with lung pathology that does not produce specific artefacts, such as asthma, COPD or pulmonary embolism.
Given the parietal and visceral pleura slide against each other when the patient breathes, the normal pleural line has a shimmery aspect that resembles ‘ants marching’. This is known as dynamic lung sliding, and its presence rules out pneumothorax. The pleural layers lie in close contact with each other and right on top of air-filled lung tissue. Because of this, ultrasound waves reaching the pleural line are reflected and bounce between the transducer and the visceral pleura, which is known as reverberation. The bouncing is interpreted in the screen as evenly spaced horizontal lines resembling the pleural line, yet in reality, they are a reverberation artefact known as A-lines.
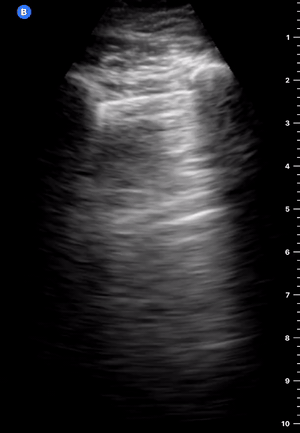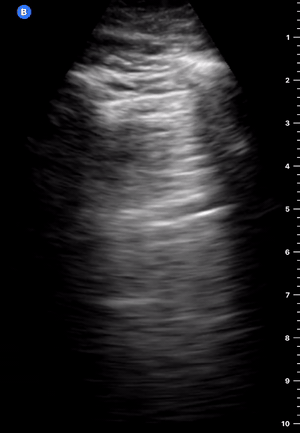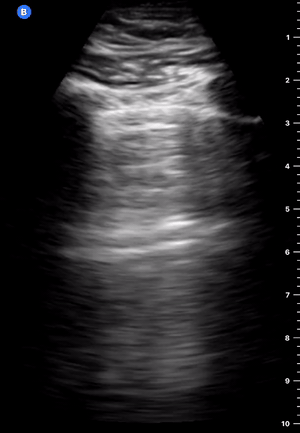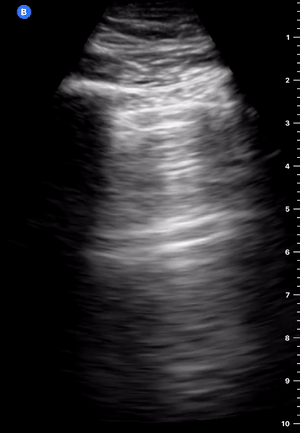
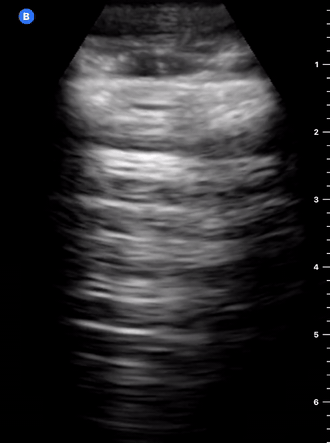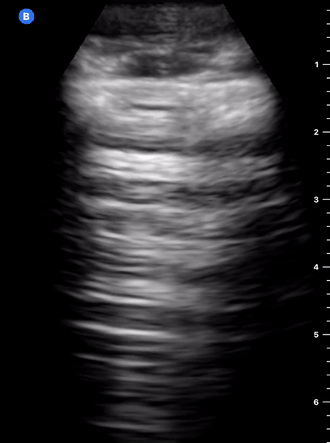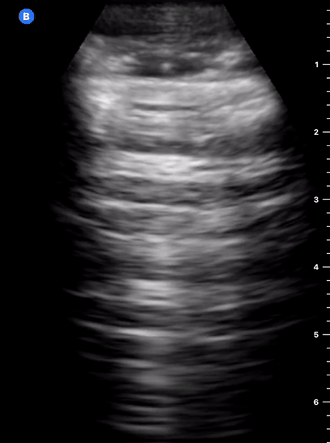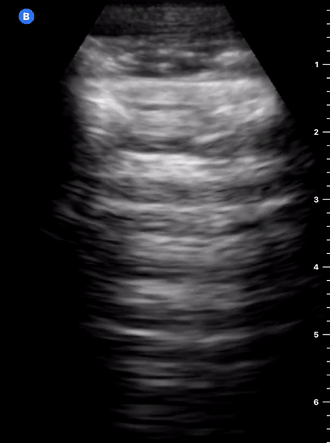
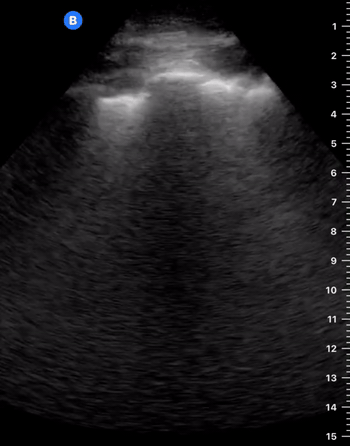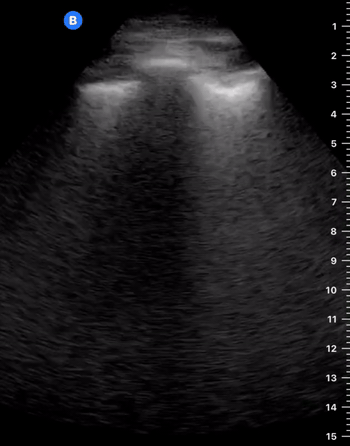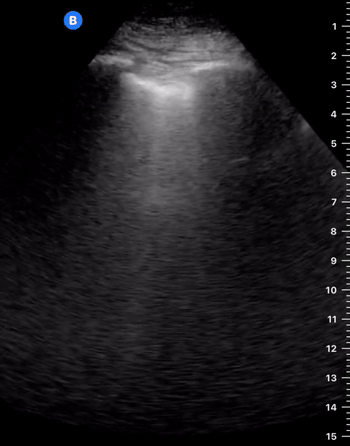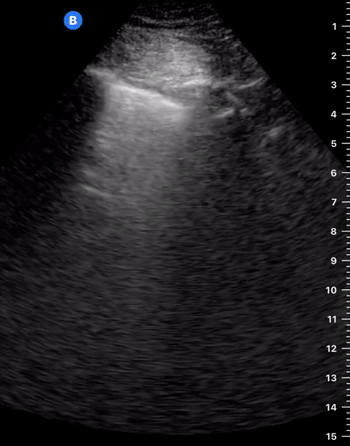
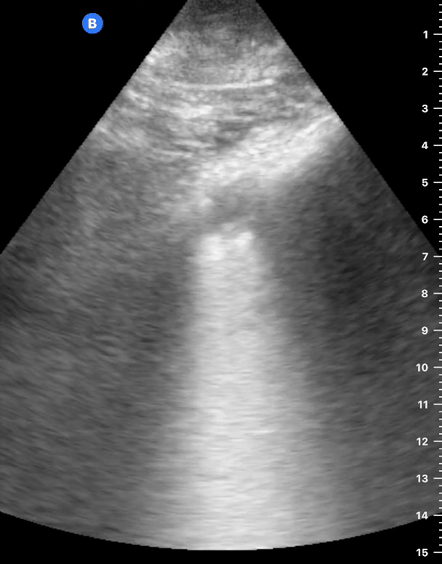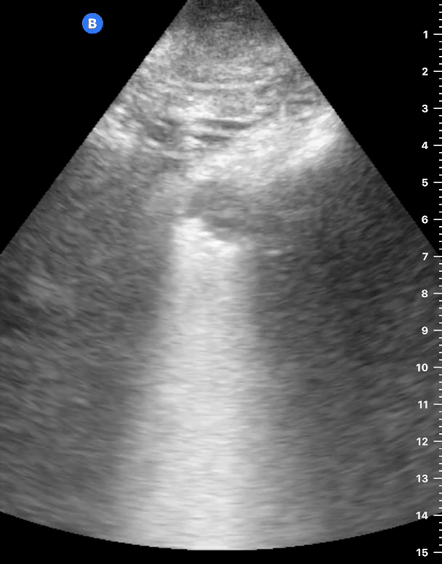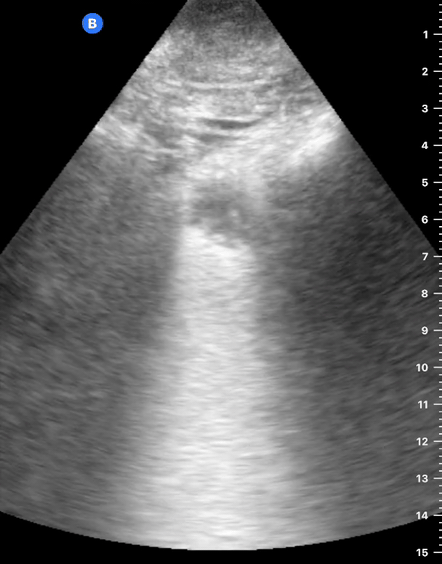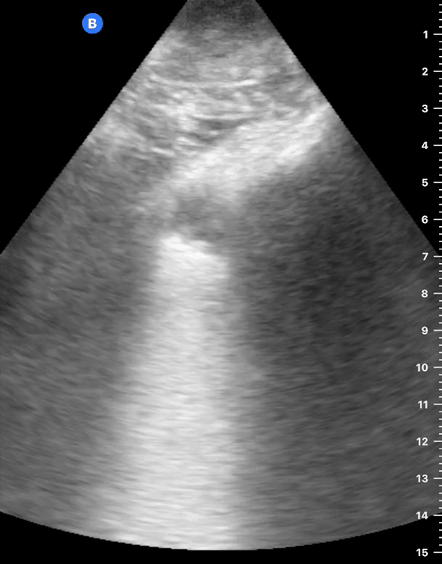
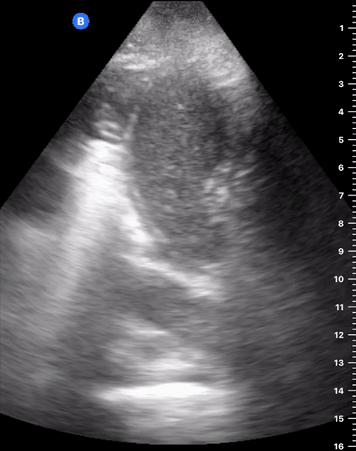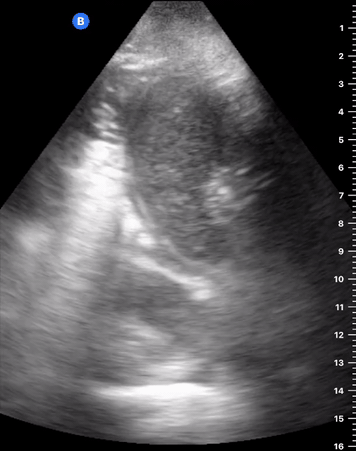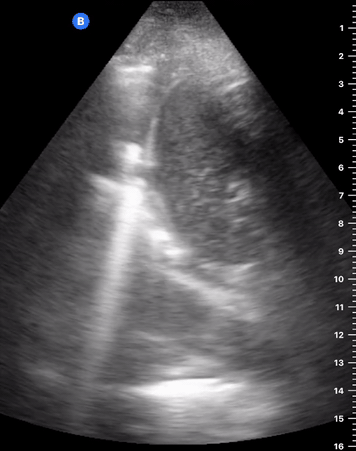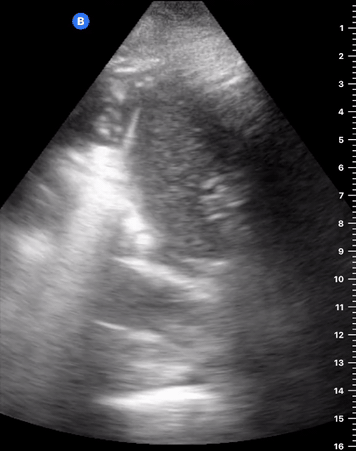
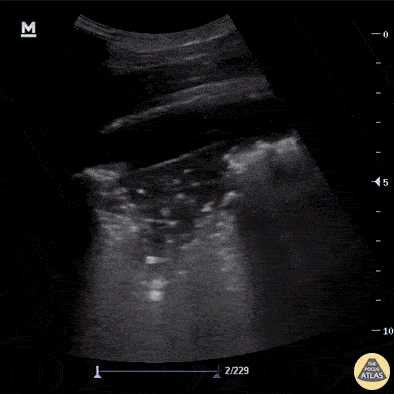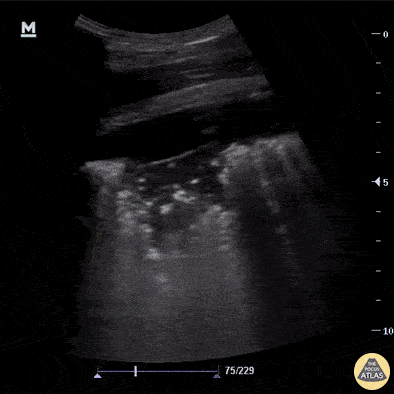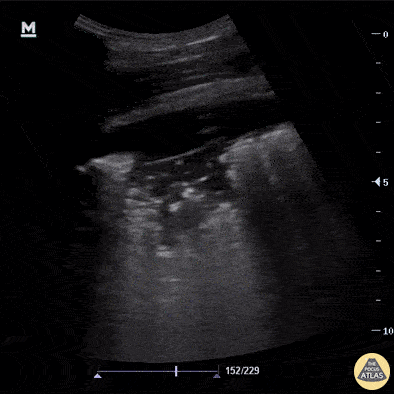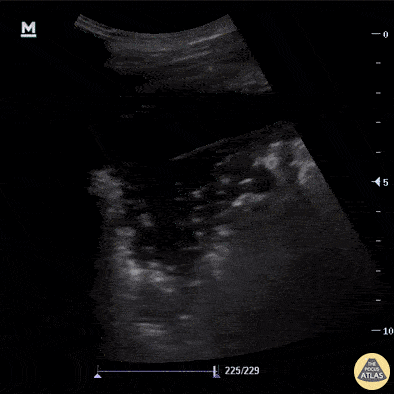
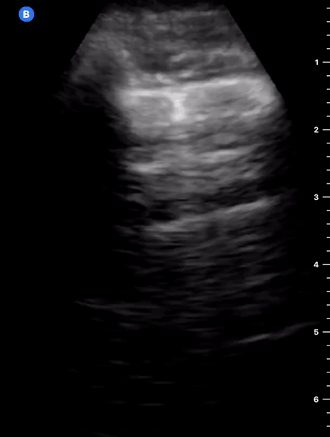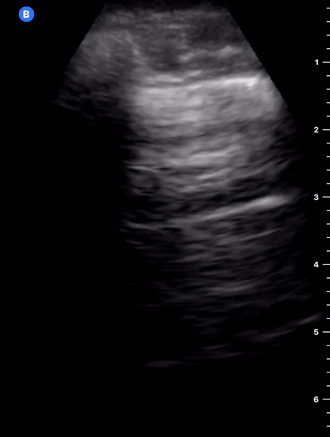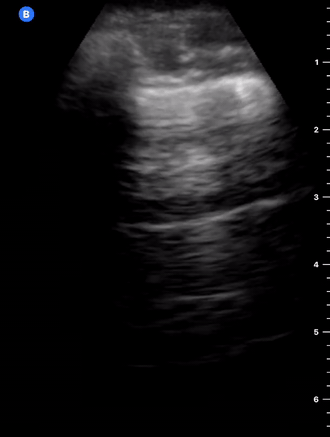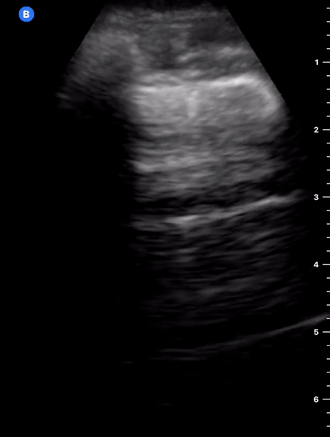
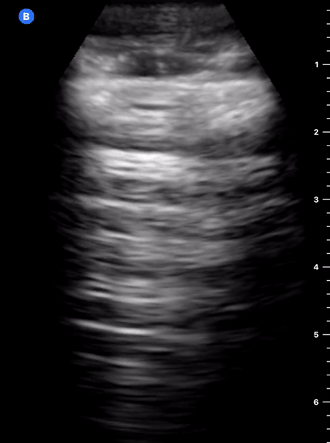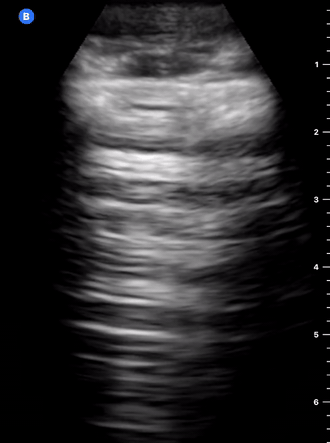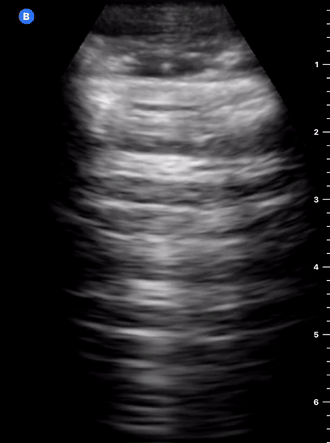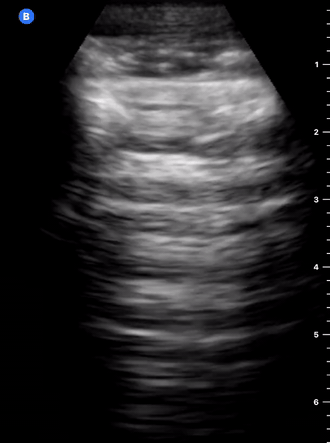
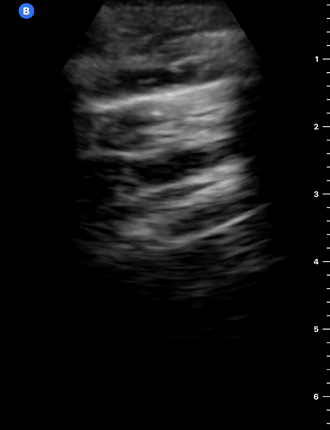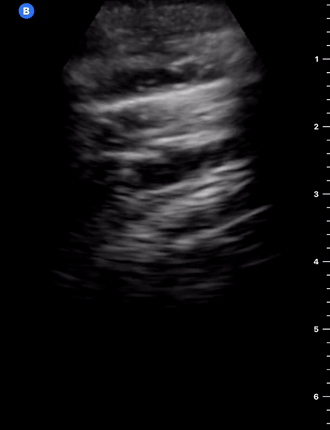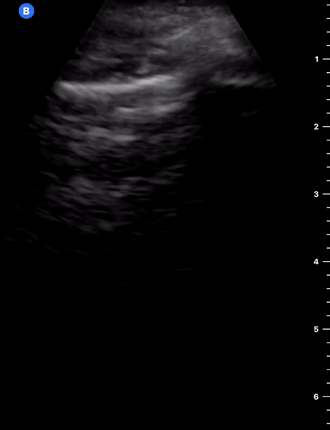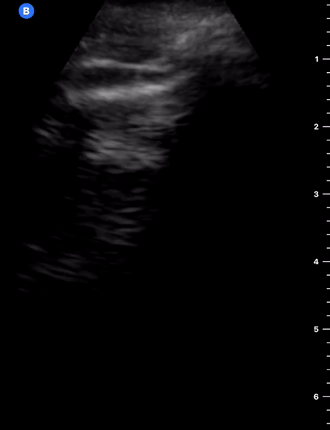
B-Profile
B-LINES
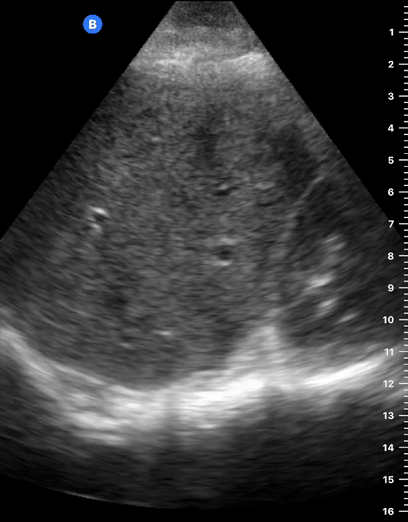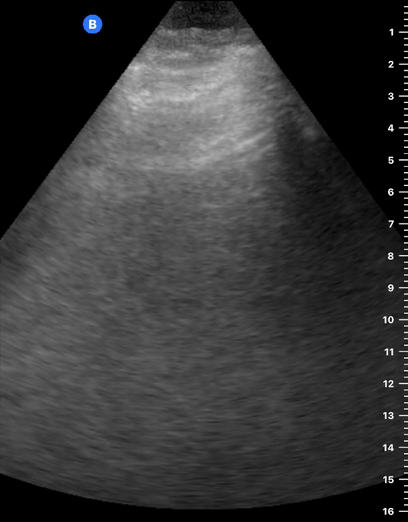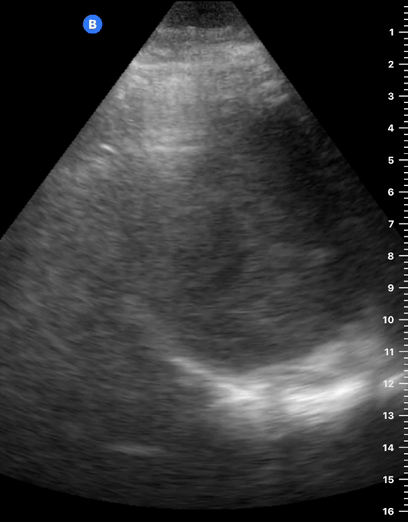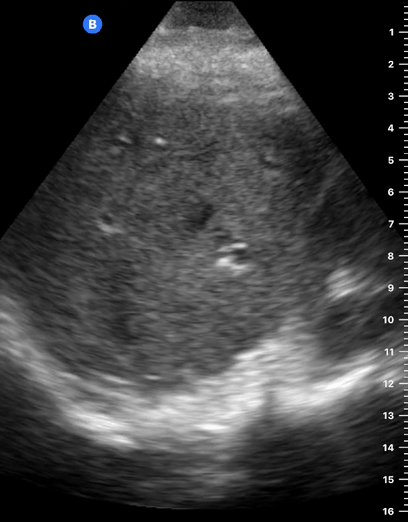
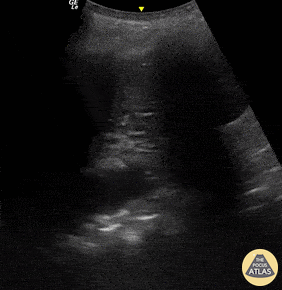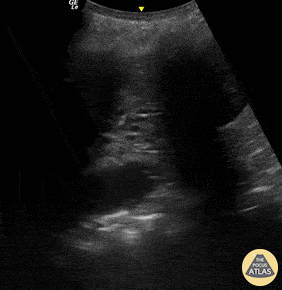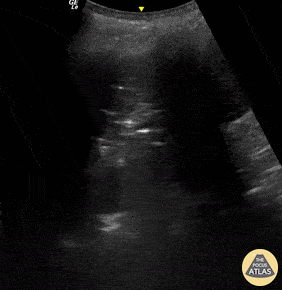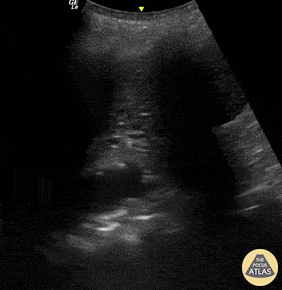
B-lines are vertical, hyperechoic reverberation artefacts secondary to air/water interfaces within the interlobular septa. B-lines can be thick or narrow; they start at the pleural line and reach the bottom of the screen, moving with respiration and obliterating the A-lines on their way. As they require pleural indemnity to exist, their presence rules out a pneumothorax.
Pathologic B-lines represent an interstitial syndrome (increased interstitial water), defined as more than two B lines between two ribs. (12) Importantly, they are also a normal finding when found at dependent areas (posterior regions in the supine patient).
B-PROFILE: LUNG ROCKETS
The presence of pathological B-lines describes a B-profile. Widespread, bilateral, and/or three or more per window are considered pathological.
Multiple B-lines in a single window are described as lung rockets because they resemble a rocket at liftoff. The magnitude of the rockets correlates with the severity of the interstitial syndrome.
IS comprises pulmonary oedema, widespread pneumonia, acute respiratory distress syndrome, alveolar haemorrhage, and chronic interstitial diseases (for example, widespread fibrosis). The diagnostic approach will depend on the clinical context and the specific features of the image. Accordingly, a breathless patient developing acute pulmonary oedema will likely show diffuse, bilateral lung rockets, whereas a patient with ARDS will have patchy, asymmetrical B-lines.
Focalized pathological lung rockets, however, represent a differential diagnosis and can be found in various other disease processes such as focal pneumonia, pulmonary contusion, pulmonary infarction, cancer, or pleural disease.
Alternatively, the presence or evolution of B-lines can be used to guide fluid resuscitation and diuresis. For example, the development of lung rockets during aggressive intravenous fluid administration should represent a warning sign to stop or slow down fluids.
C-Profile
IS THERE PNEUMONIA OR ATELECTASIS?
Although many guidelines still recommend the chest X-ray as the initial study to rule out pneumonia, it is clear that its sensitivity (75%) and diagnostic accuracy are low when compared to ultrasound. The lung US has better sensitivity (92%) and specificity (93%) for consolidation (6), and it can also provide a differential diagnosis in expert hands. In case there is a consolidation that extends to the pleura, as is the case 98% of the time, it will be possible to visualize it by ultrasound (11). Yet, sonography cannot detect areas of consolidation deep into normal lung tissue, and, as a result, it cannot be used to rule out consolidation. Given that consolidation can arise at any site of the lung, ultrasound sensitivity is dependent on the site, size, and time spent scanning.
CONSOLIDATION
“If you can see the structural anatomy of lung tissue, then it is abnormal. Put simply; a non-aerated lung resembles the tissue of solid organs such as the liver. This can occur in several disease states such as collapse, consolidation, contusion, atelectasis and malignancy” (1). When scanning the lung bases, however, be aware that owing to the curved and reflective surface of the diaphragm, mirror artefacts of the liver can be seen in the normal lung area, which should not be confused with consolidation.
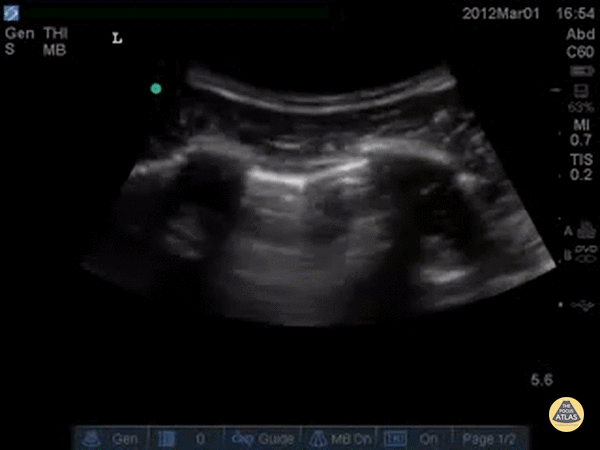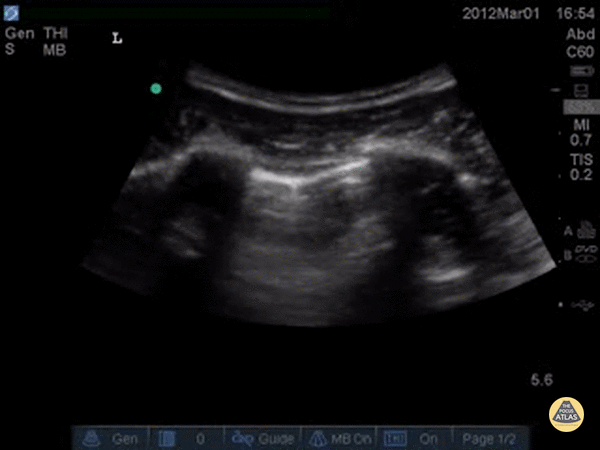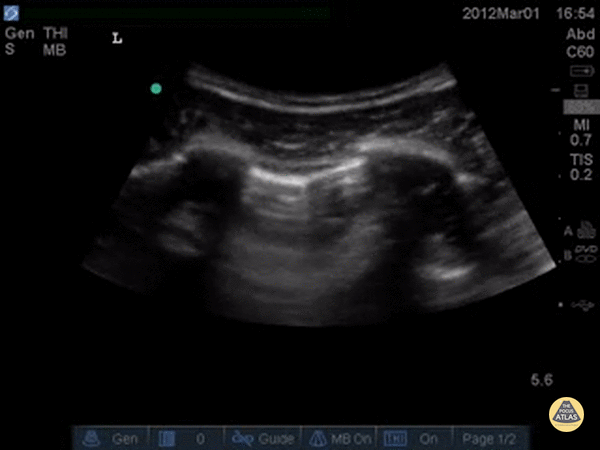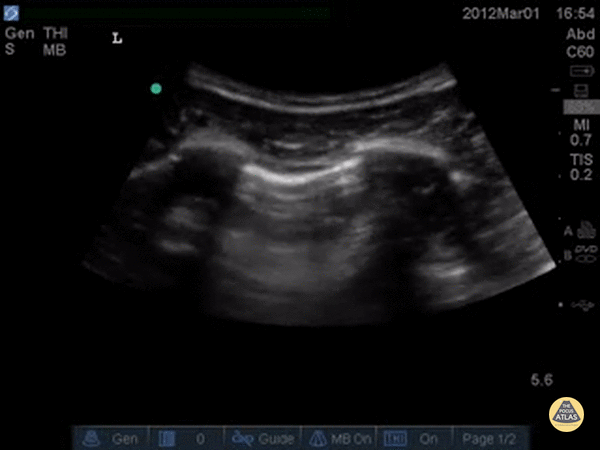
SHRED SIGN & AIR BRONCHOGRAM
Nontranslobar consolidations (the most common) are identified by the shred sign, where the border between the consolidated and aerated lung is irregular, drawing the fractal line. The sign indicative of translobar consolidation is the tissue-like sign (lung hepatisation). Both signs allow 90% sensitivity and 98% specificity for consolidation. The presence of pulmonary hepatisation associated with dynamic air bronchograms and focal B-lines confirms the diagnosis of pneumonia at the patient’s bedside. On the other hand, static air bronchograms are characteristic of atelectasis.
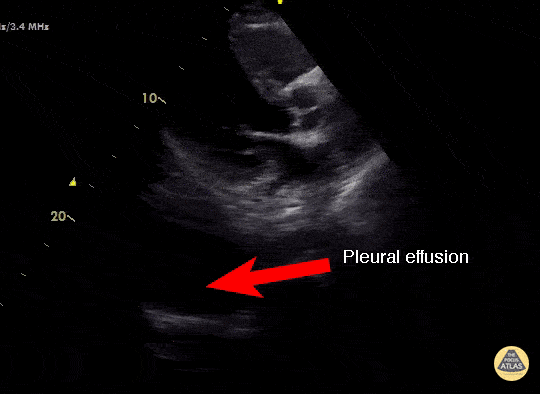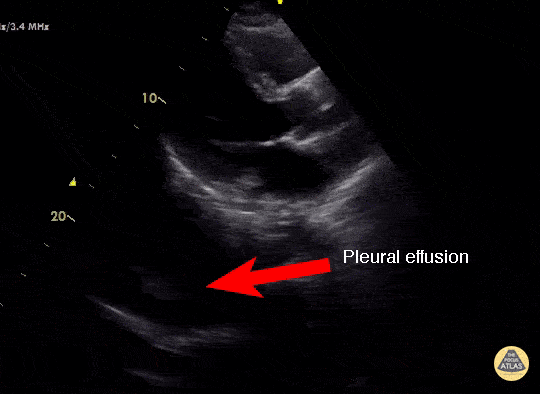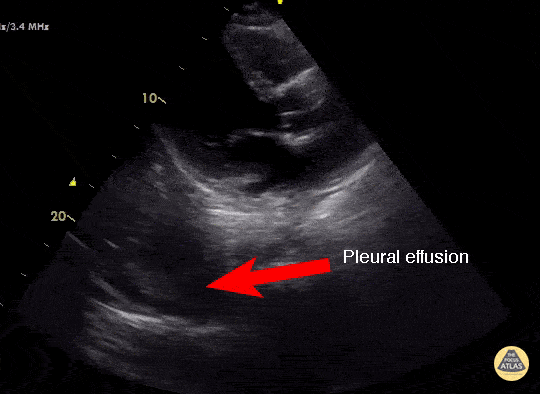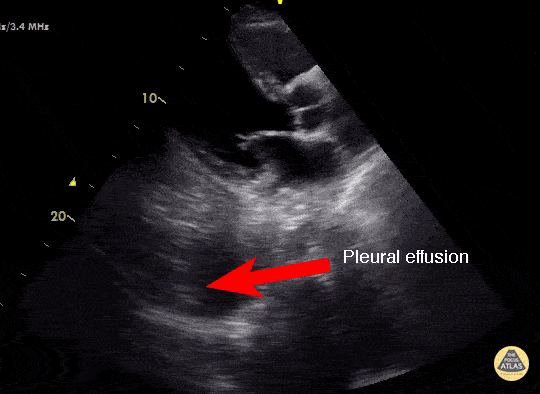
Effusion
IS THERE PLEURAL EFFUSION?
Pleural effusion collects in the most dependent areas of the lung, particularly in the costophrenic angles. Its aspect can vary from black/anechoic (fresh blood, transudate/exudate) to grey/echogenic (e.g. clotted blood, exudate).
LUNG CURTAIN & SPINE SIGN
When scanning the base of a normal lung, we would notice how the hyperechoic spine at the bottom of the screen is abruptly interrupted by the diaphragm, and the normal respiratory movement, as a ‘lung curtain’, would obscure the image during inspiration. By contrast, the presence of fluid in the thorax and costophrenic angles will transmit US waves deeper into the body, allowing visualization of thoracic vertebrae beyond the diaphragm (‘spine sign’). Also, as it no longer forms an interface with the reflective air-filled lung, the diaphragm appears less echogenic.
FLUID DIFFERENTIALS
In case there is a large amount of fluid, the collapsed lung can be seen floating within; this is known as ‘jellyfish sign’ or ‘elephant trunk sign’. Also, when scanning the heart in the left hemithorax, it can be difficult to differentiate pleural fluid from pericardial effusion. Pericardial fluid passes in front of the descending aorta, while pleural fluid surrounds the descending aorta. Similarly, peritoneal fluid can be mistaken for pleural fluid. In this case, the key is to carefully identify the diaphragm, as pleural collections are superior to it.
Pneumothorax
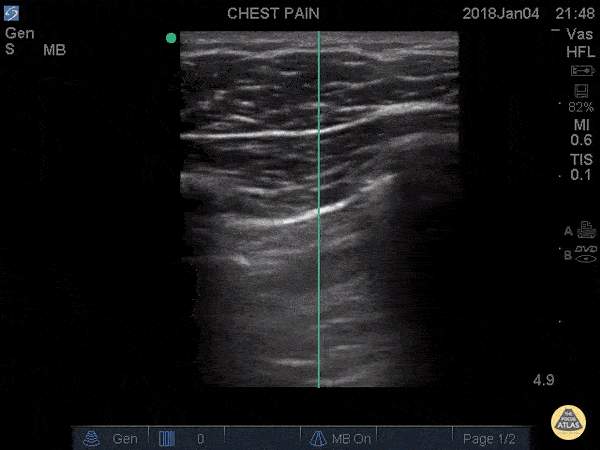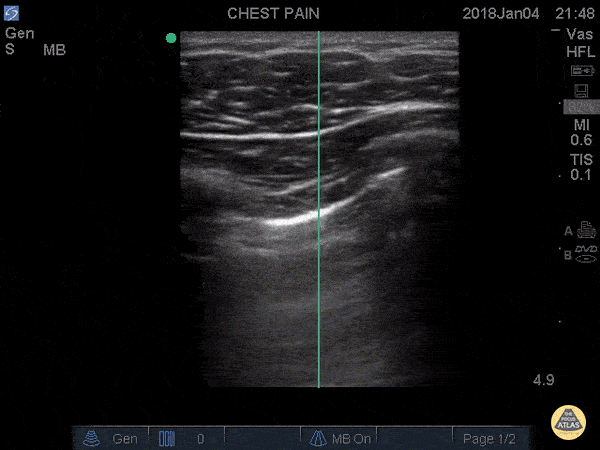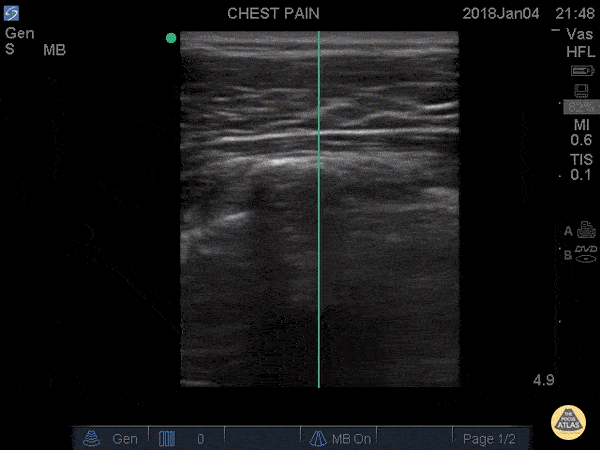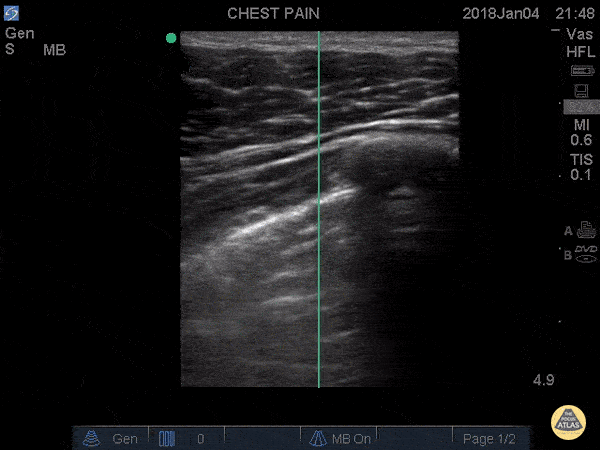
IS THERE LUNG SLIDING?
This profile is characterised by absent lung sliding and present A-lines. The finding of dynamic lung sliding rules out the presence of pneumothorax. In addition, the finding of the B lines artefact indicates indemnity of both pleural layers and rules out the presence of pneumothorax.
When there is a pneumothorax, the pleural layers are separated by air. Ultrasound waves are reflected at the encounter of the parietal pleura, which lies upon the air of the pneumothorax itself. Therefore, movement of the visceral pleura and lung deep to the pneumothorax cannot be seen, leading to loss of the typical shimmering, dynamic appearance.
ABSENT LUNG SLIDING
Although it is usually a key feature of pneumothorax, the absence of dynamic lung sliding lacks specificity and can also be found in other conditions such as apnea, atelectasis, bullae, chronic airflow limitation, pleurodesis, inflammatory adherences, fibrosis, right main-stem intubation, oesophagal intubation and cardiopulmonary arrest.
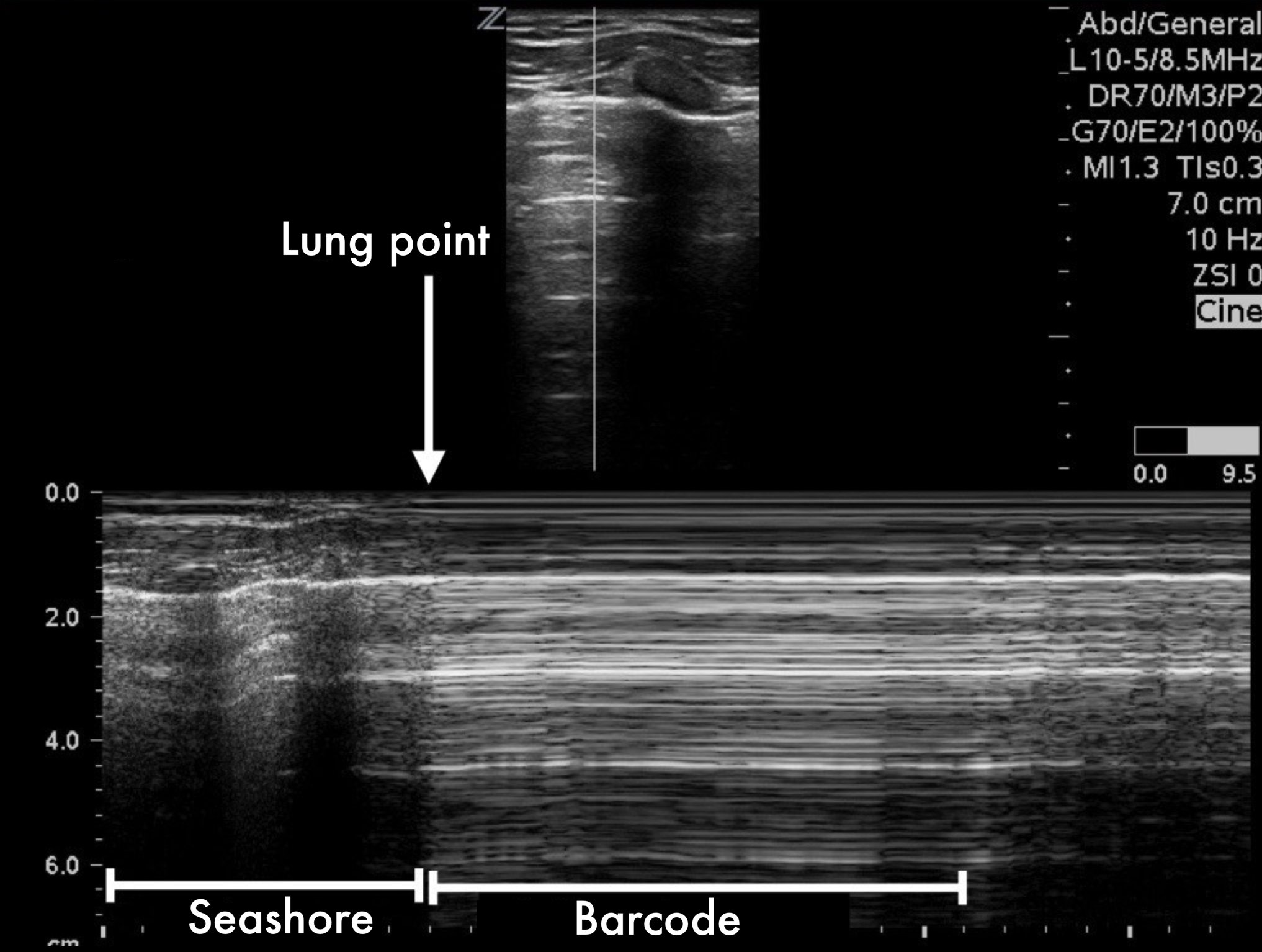
LUNG POINT
Conversely, the lung point sign is pathognomonic and 100% specific for pneumothorax. It represents the transition point where a normal lung gives way to pneumothorax: sliding is present on one side of the image while absent on the other. Additionally, the lung point indicates pneumothorax volume: moderate if anterior, massive if posterior or absent. Lateral lung points correlate with a 90% need for drainage versus 8% with anterior lung-point (11).
The M mode is far from essential but can be helpful in the interpretation if you struggle to identify sliding in B-Mode. A ‘seashore image’ will be seen in the normal lung, whereas a ‘barcode image’ will appear in absence of lung sliding. Remember, absent lung sliding by itself is not diagnostic of pneumothorax.
Bedside Lung Ultrasound in Emergency: BLUE
This is a modification from Lichtenstein's original algorithm. The BLUE-protocol decision tree illustrates a diagnostic approach for the main causes of undifferentiated dyspnoea / acute respiratory failure. Notice that it includes a venous ultrasound scan. The PLAPS point is an addition to the diagram, as it represents the most sensitive point for finding effusion and consolidation regardless of the presence of lung sliding. Effusion is often found accompanying other disease processes. In this way, both Lung Sliding (anterior and lateral chest wall) and PLAPS point are entry doors to the diagram leading to or suggesting a diagnosis. Asthma/COPD is possible after having considered the other causes.
Adapted by Felipe Urriola from ‘Lung Ultrasound in the Critically Ill’ (11).
Taken from ‘Lung Ultrasound in the Critically Ill’ (11).
Areas of investigation and the BLUE-points. Two hands placed this way (size equivalent to the patient’s hands, upper hand touching the clavicle, thumbs excluded) correspond to the location of the lung, and allow three standardized points to be defined. The upperBLUE-point is at the middle of the upper hand. The lower-BLUE-point is at the middle of the lower palm. The PLAPS-point is defined by the intersection of: a horizontal line at the level of the lower BLUE-point; a vertical line at the posterior axillary line. Small probes, such as this Japanese microconvex one (1992), allow positioning posterior to this line as far as possible in supine patients, providing more sensitive detection of posterolateral alveolar or pleural syndromes (PLAPS). The diaphragm is usually at the lower end of the lower hand (11).
Author: Felipe Urriola Perez | Published: 2 December 2022
Reference.
Bowra, Justin; McLaughlin, Russell; Atkinson, Paul; Henry, Jaimie. Emergency Ultrasound Made Easy, Third Edition. 2022. Elsevier Health Sciences.
Basaure, Carlos; Clausdorff, Hans; Riquelme; Felipe. Ultrasonido Clínico, First edition. 2018. Emergency Medicine Residency Program, Universidad Católica de Chile.
Alrajhi K, Woo MY, Vaillancourt C. Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta-analysis. Chest. 2012 Mar;141(3):703-708. 10.1378/chest.11-0131
Yousefifard M, Baikpour M, Ghelichkhani P, Asady H, Shahsavari Nia K, Moghadas Jafari A, Hosseini M, Safari S. Screening Performance Characteristic of Ultrasonography and Radiography in Detection of Pleural Effusion; a Meta-Analysis. Emerg (Tehran). 2016 Winter;4(1):1-10. PMC4744606
Al Deeb M, Barbic S, Featherstone R, Dankoff J, Barbic D. Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: a systematic review and meta-analysis. Acad Emerg Med. 2014 Aug;21(8):843-52. 10.1111/acem.12435
Orso D, Guglielmo N, Copetti R. Lung ultrasound in diagnosing pneumonia in the emergency department: a systematic review and meta-analysis. Eur J Emerg Med. 2018 Oct;25(5):312-321. 10.1097/MEJ.0000000000000517
Peng, Q., Wang, X. & Zhang, L. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic. Intensive Care Med 46, 849–850 (2020). PMC7080149
Huang, Yi and Wang, Sihan and Liu, Yue and Zhang, Yaohui and Zheng, Chuyun and Zheng, Yu and Zhang, Chaoyang and Min, Weili and Zhou, Huihui and Yu, Ming and Hu, Mingjun, A Preliminary Study on the Ultrasonic Manifestations of Peripulmonary Lesions of Non-Critical Novel Coronavirus Pneumonia (COVID-19) (February 26, 2020). https://dx.doi.org/10.2139/ssrn.3544750
Pivetta, Emanuele, Alberto Goffi, Maria Tizzani, Stefania M. Locatelli, Giulio Porrino, Isabel Losano, Dario Leone, et al. 2020. “Lung Ultrasonography for the Diagnosis of SARS-CoV-2 Pneumonia in the Emergency Department.” Annals of Emergency Medicine, October. 10.1016/j.annemergmed.2020.10.008
Lichtenstein, Daniel; Mezière, Gilbert. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest. 2008 Jul;134(1):117-25. doi: 10.1378/chest.07-2800. Epub 2008 Apr 10. Erratum in: Chest. 2013 Aug;144(2):721. PMID: 18403664; PMCID: PMC3734893.
Lichtenstein, Daniel. Lung ultrasound in the critically ill. Ann Intensive Care. 2014;4(1):1. Published 2014 Jan 9. doi:10.1186/2110-5820-4-1 PMID: 24401163
Lichtenstein, Daniel. Current Misconceptions in Lung Ultrasound: A Short Guide for Experts. Chest, 156:1, 2019, 21-25, https://doi.org/10.1016/j.chest.2019.02.332.