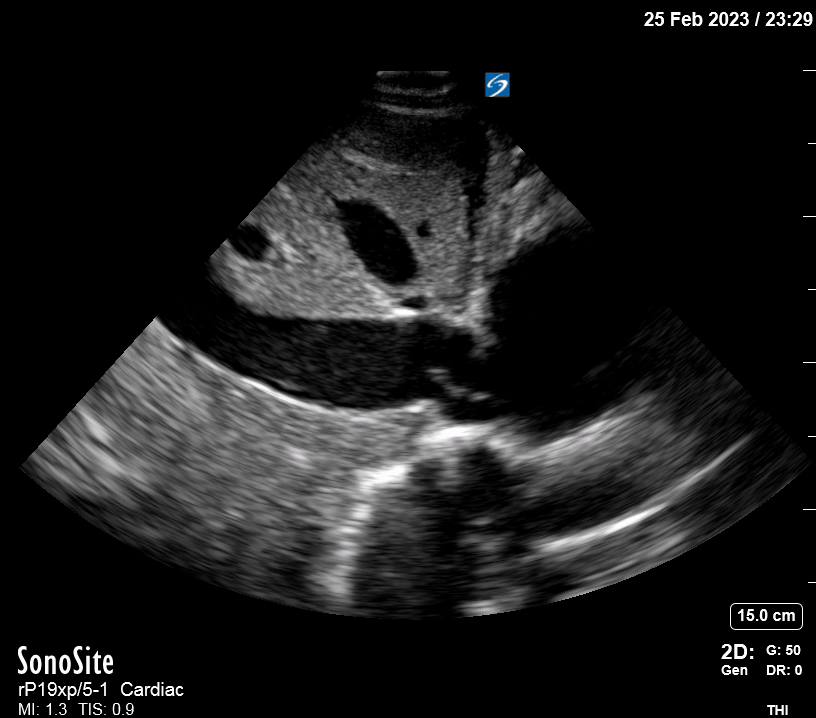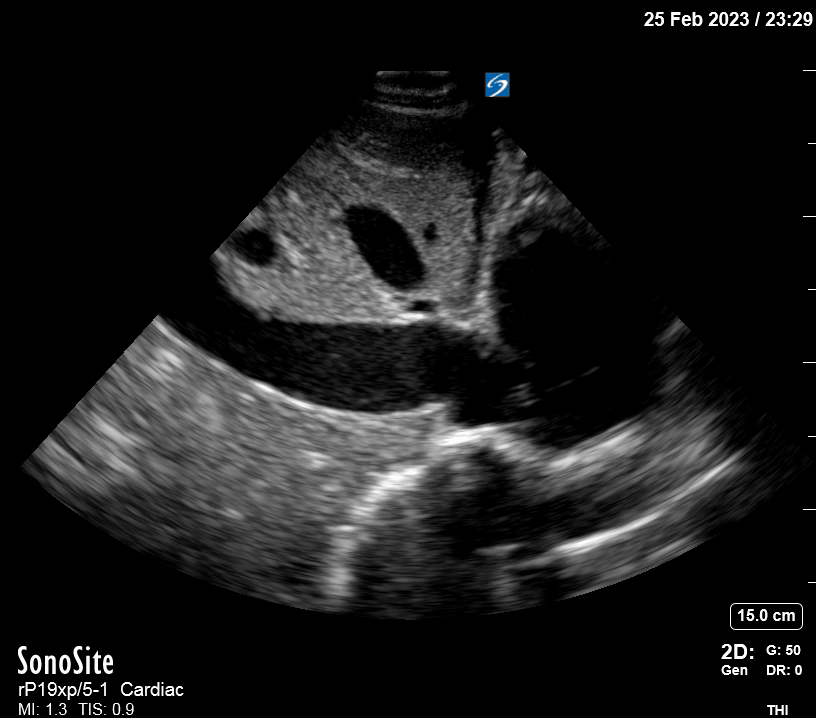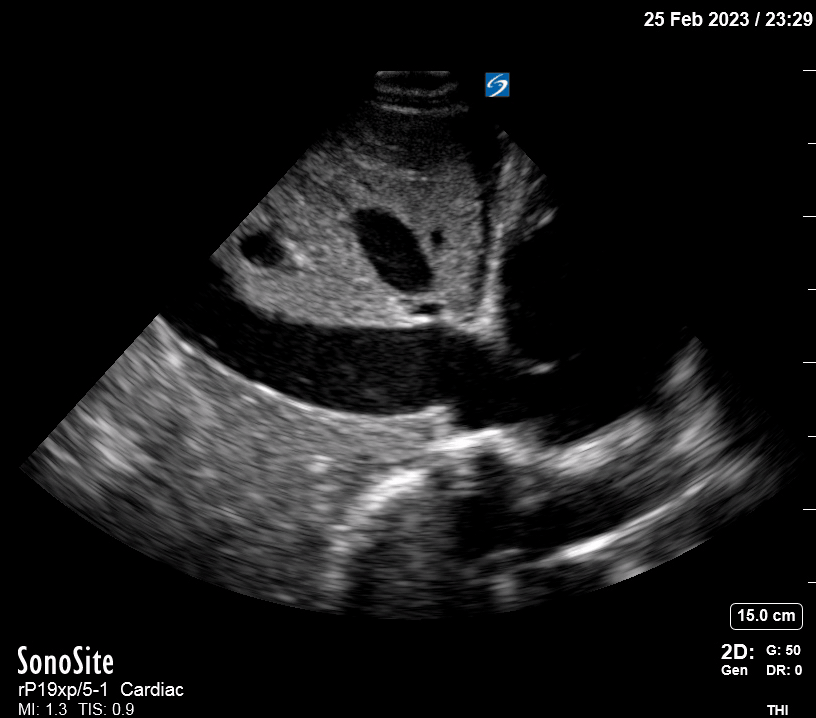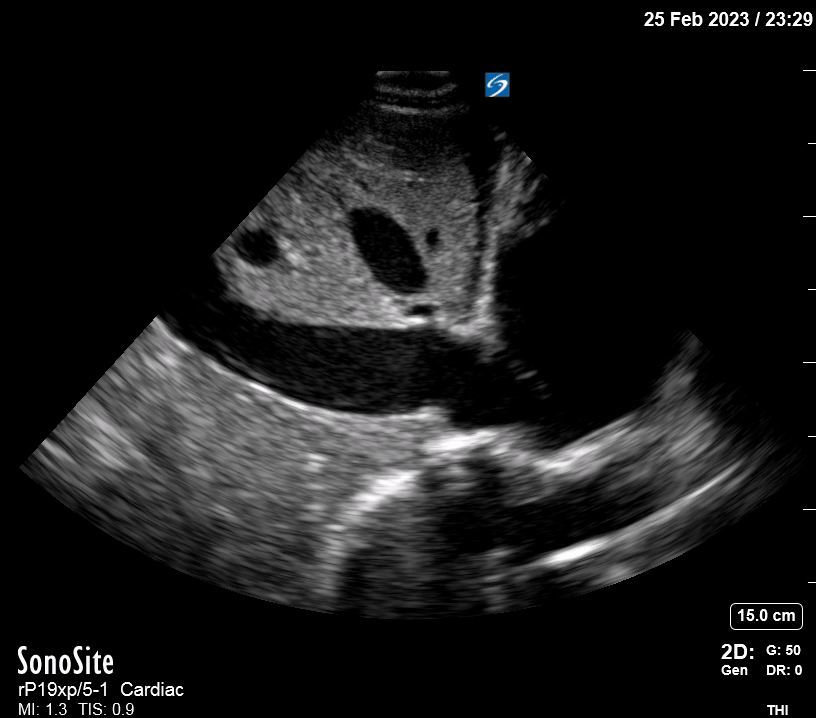
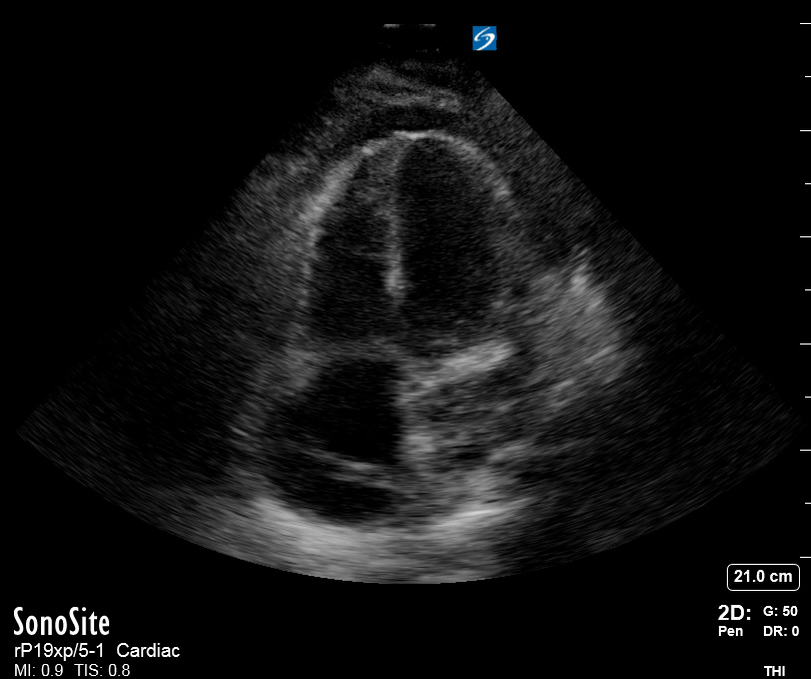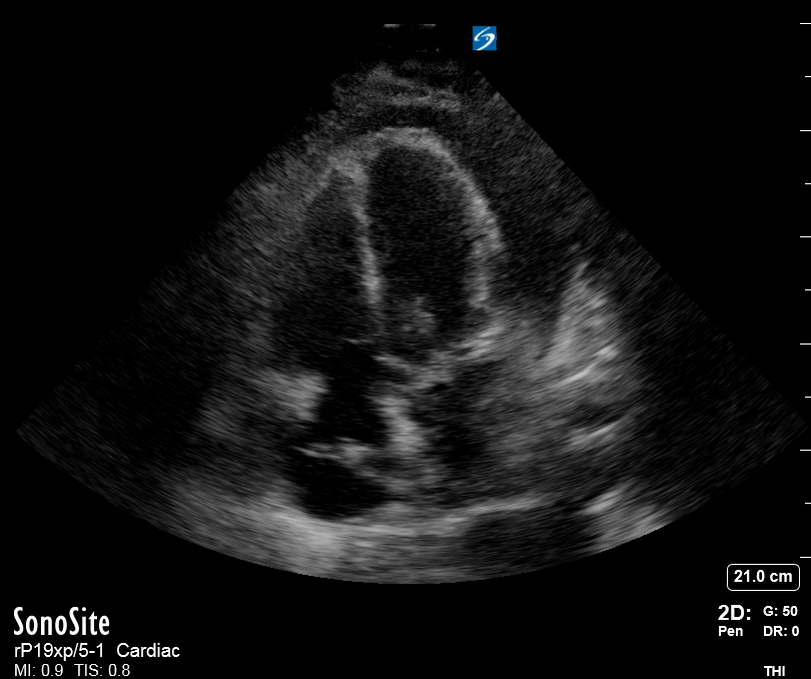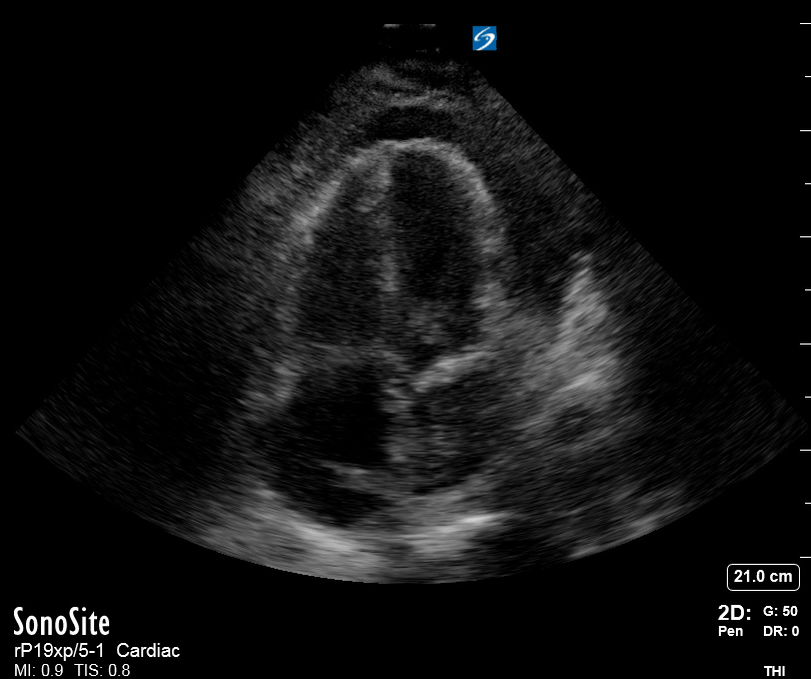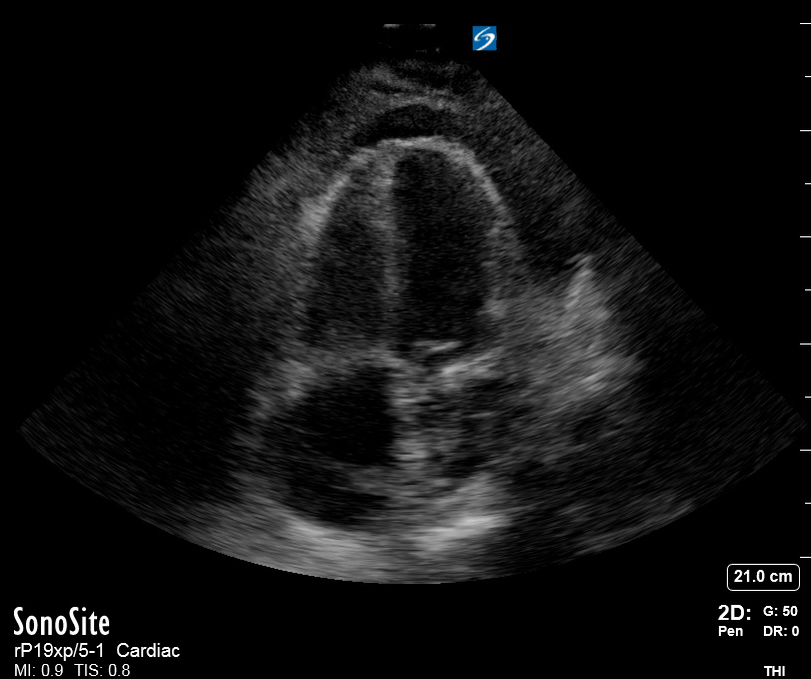
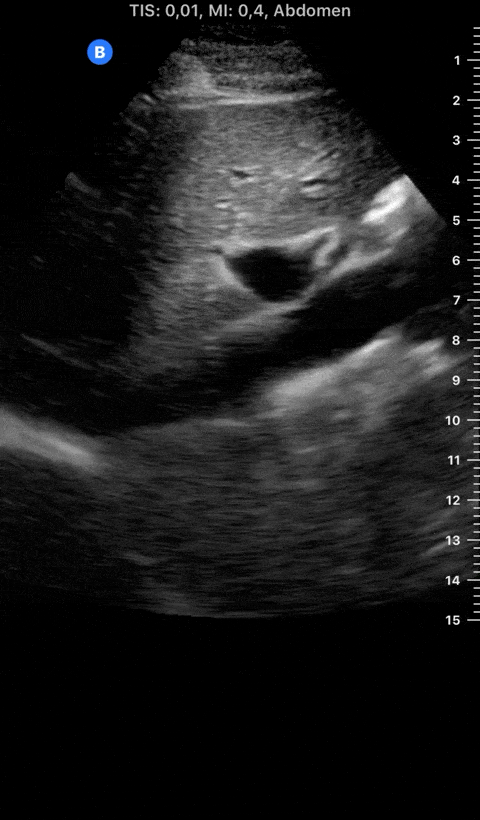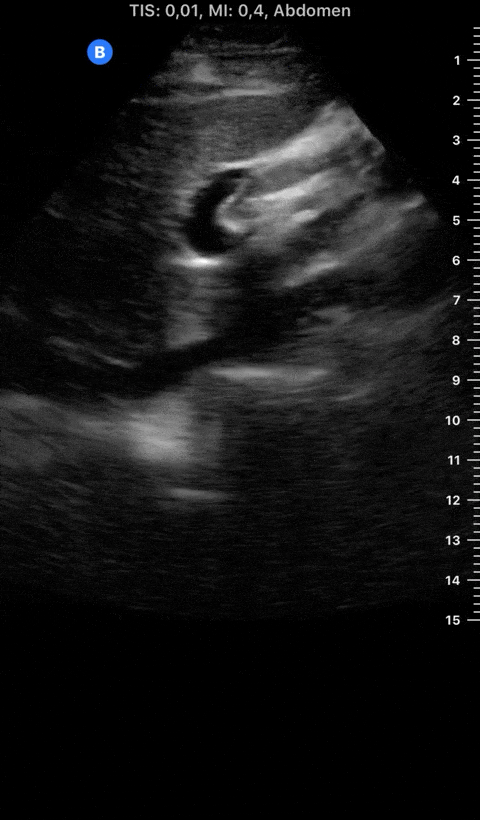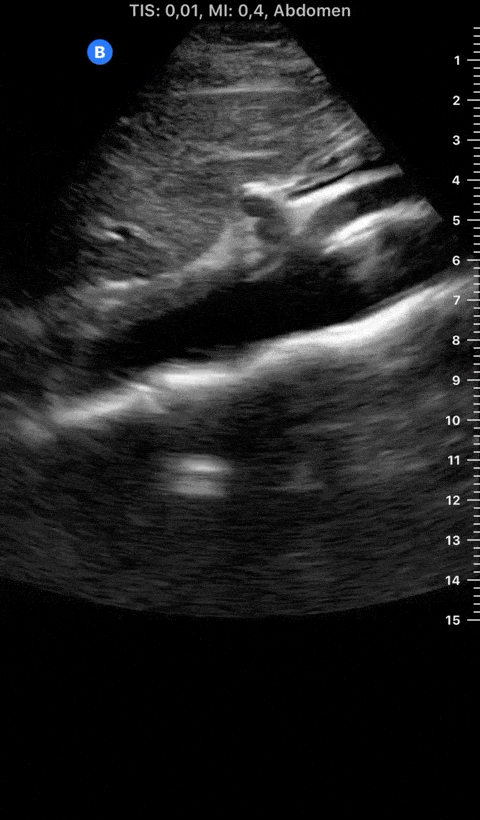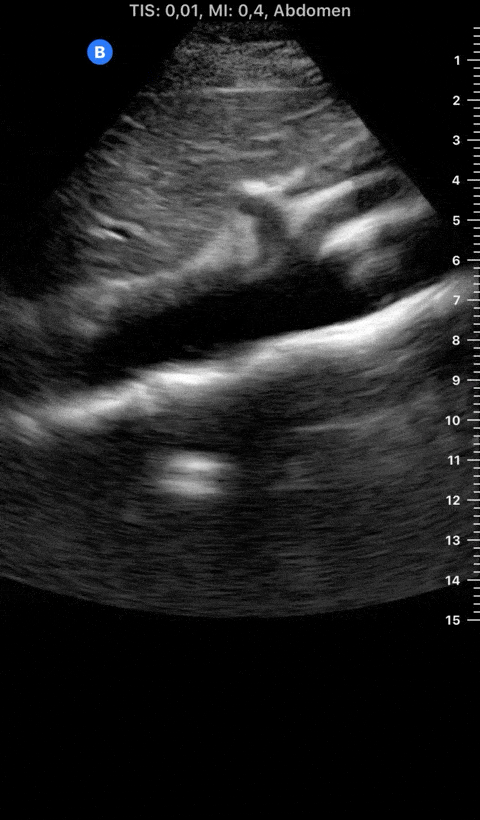
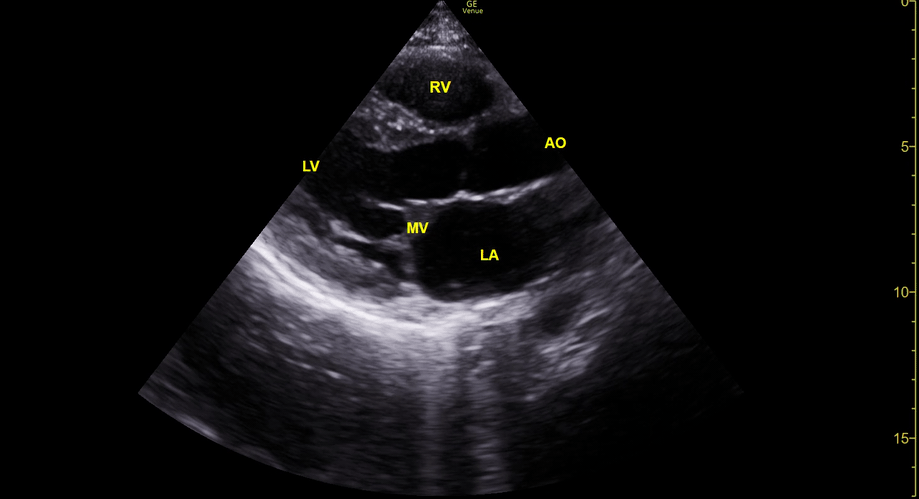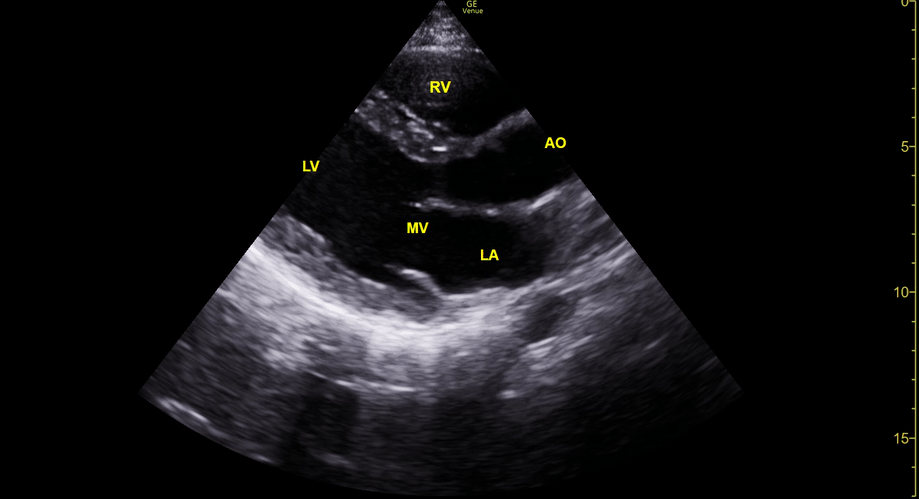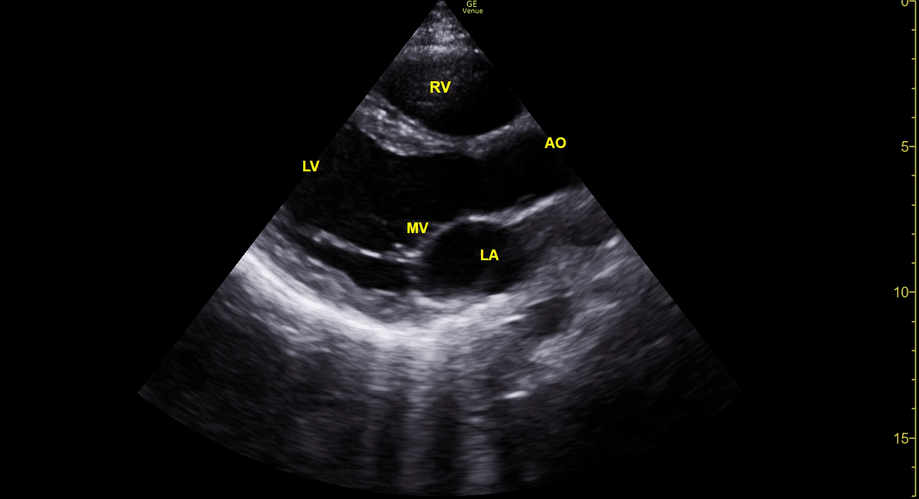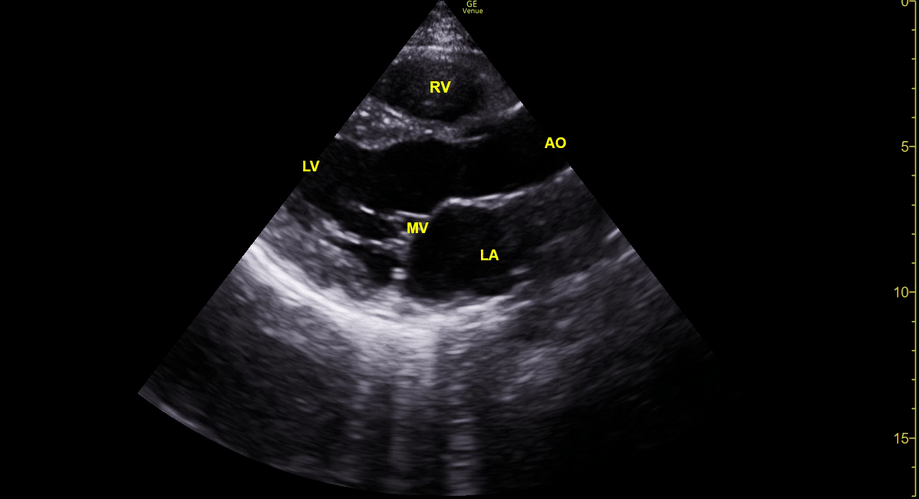
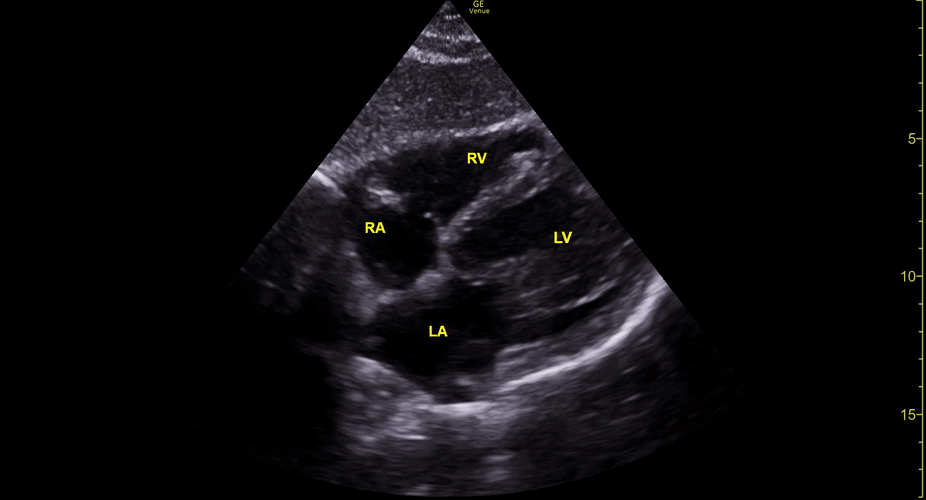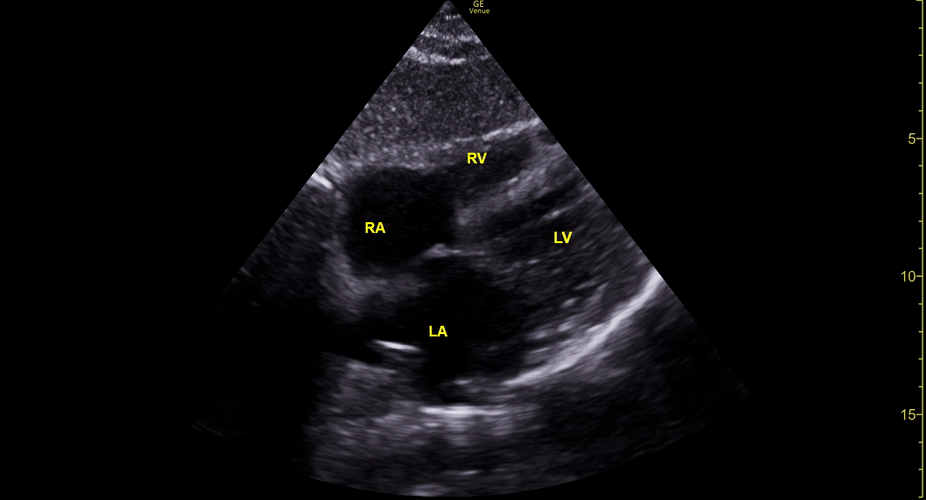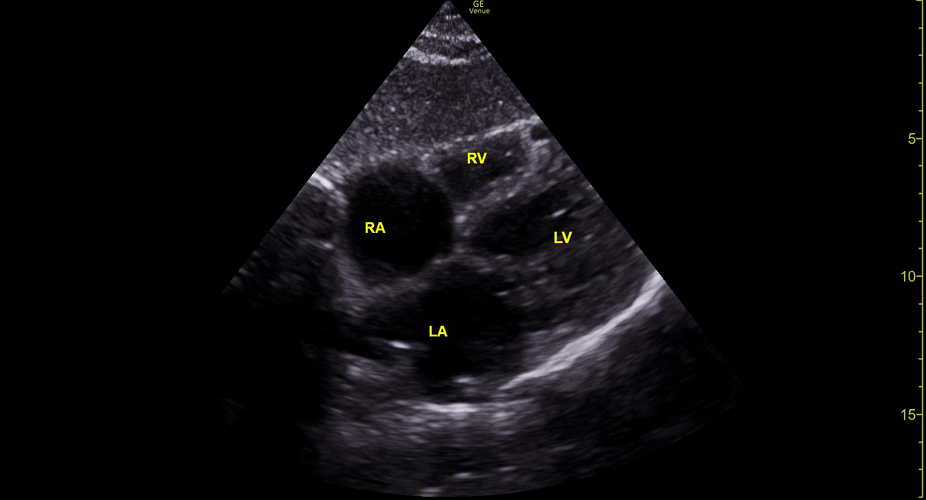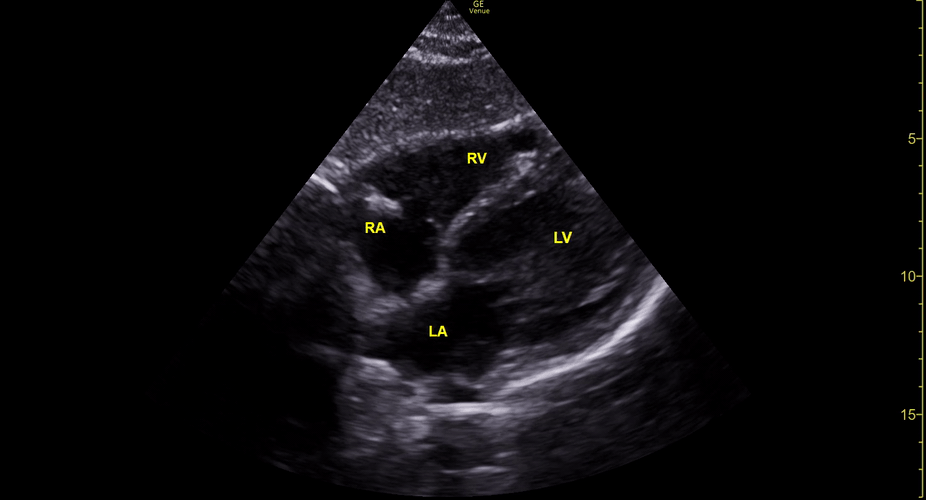
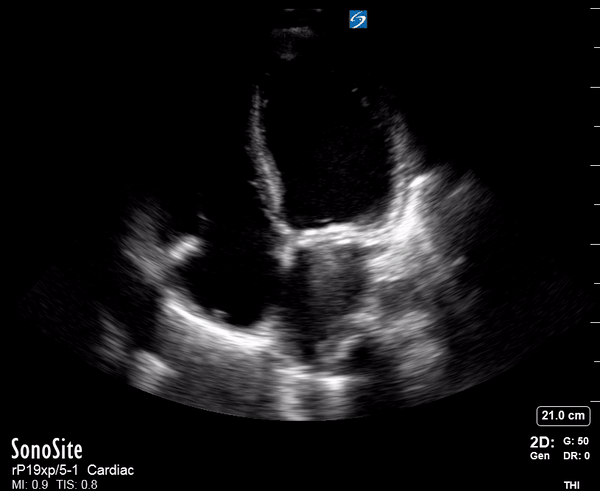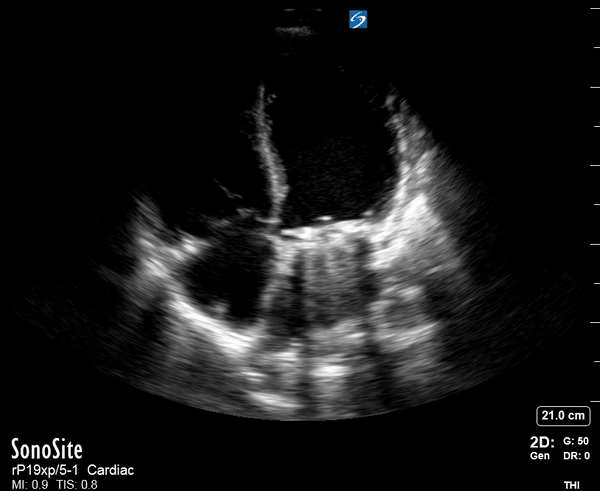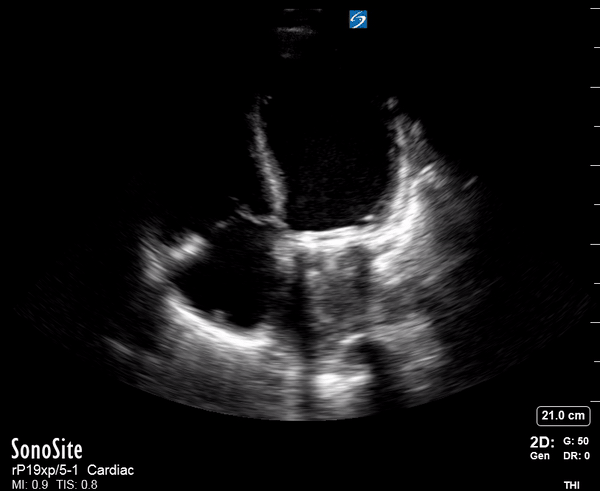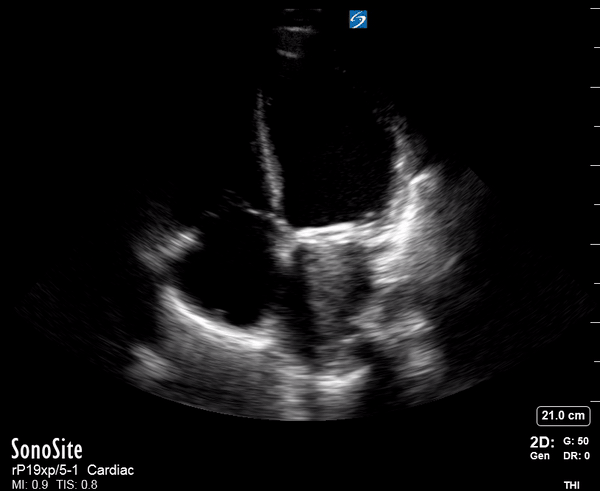
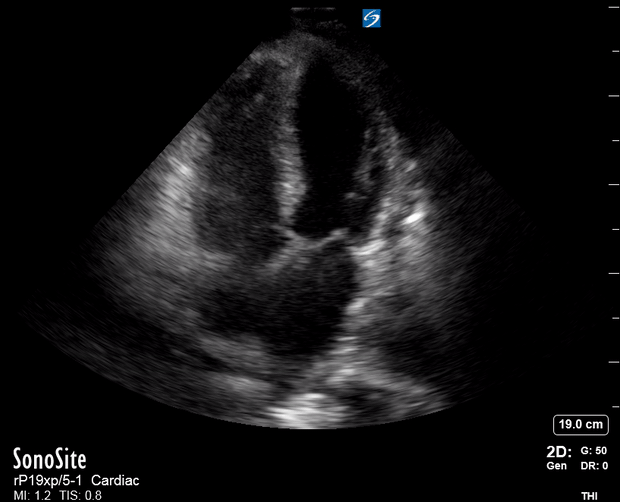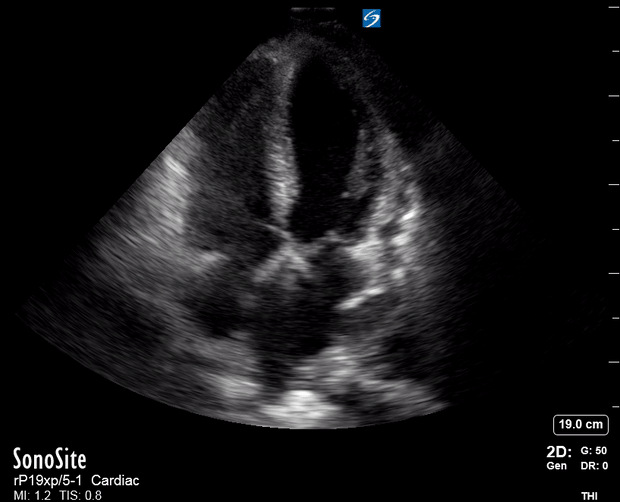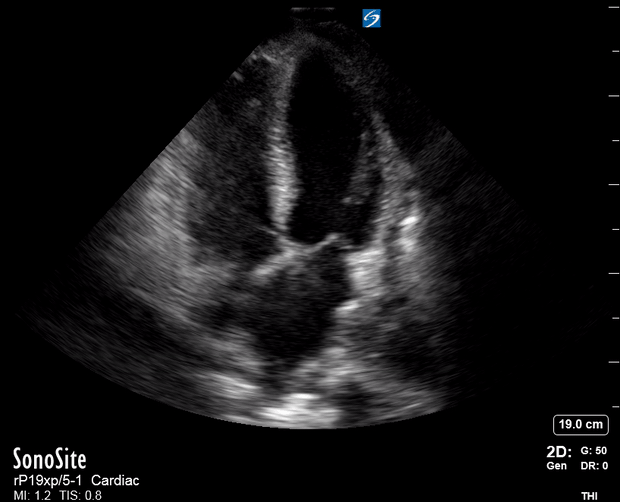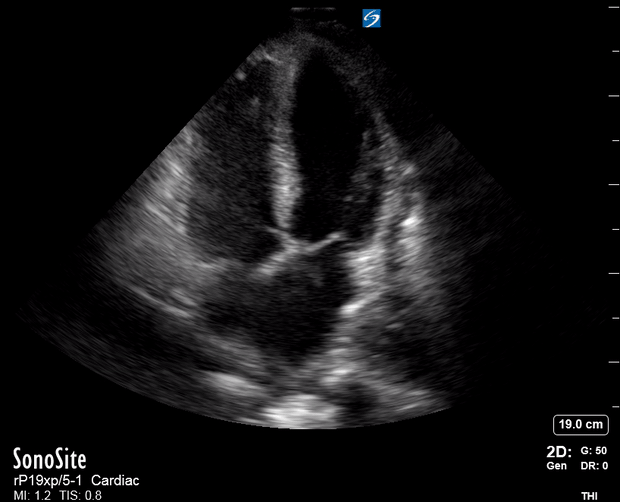
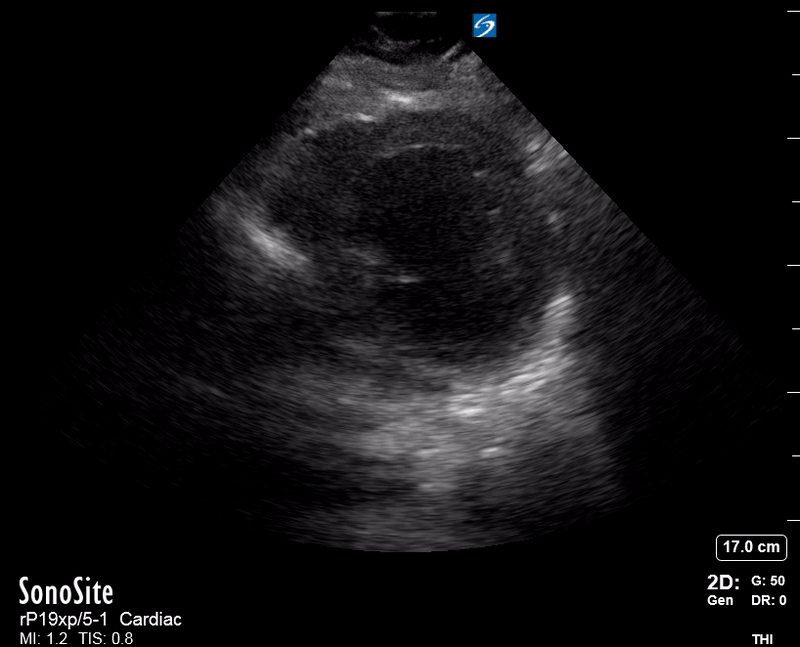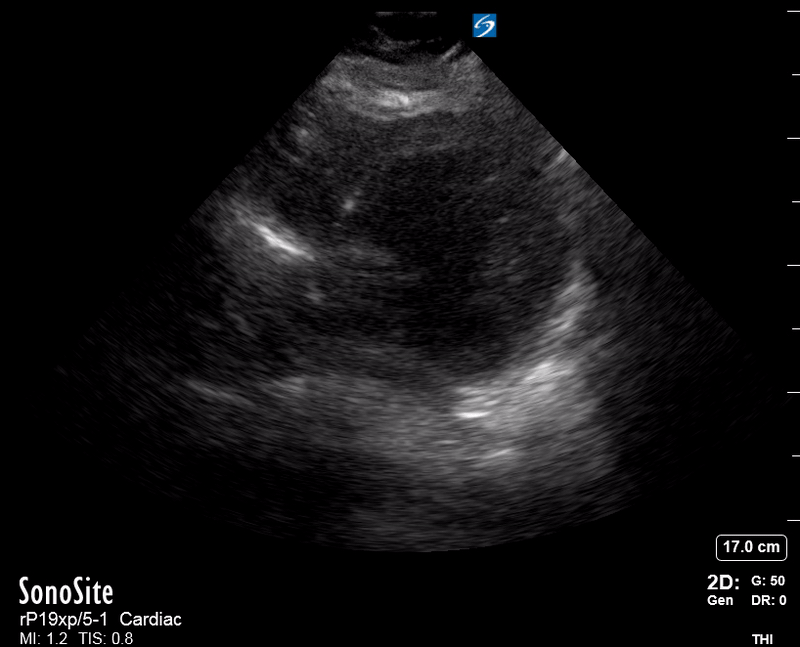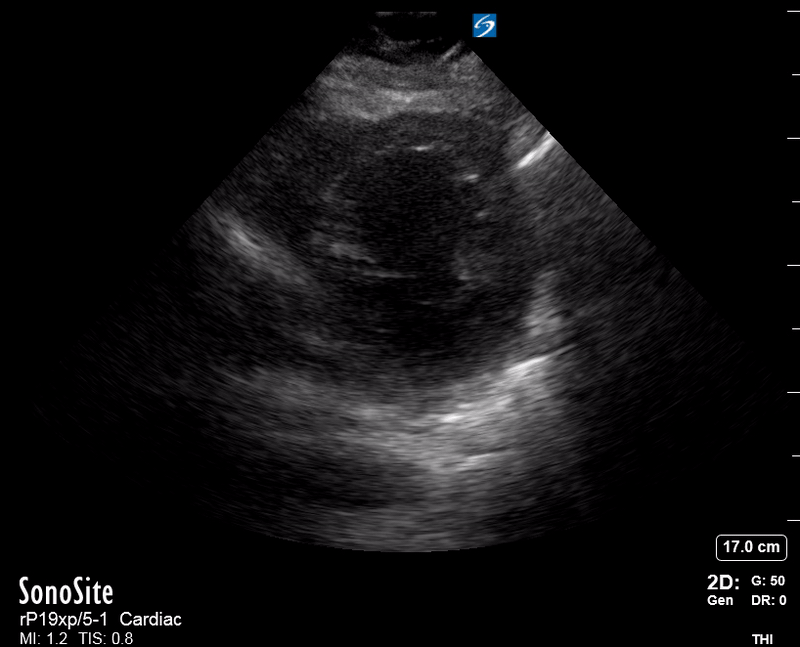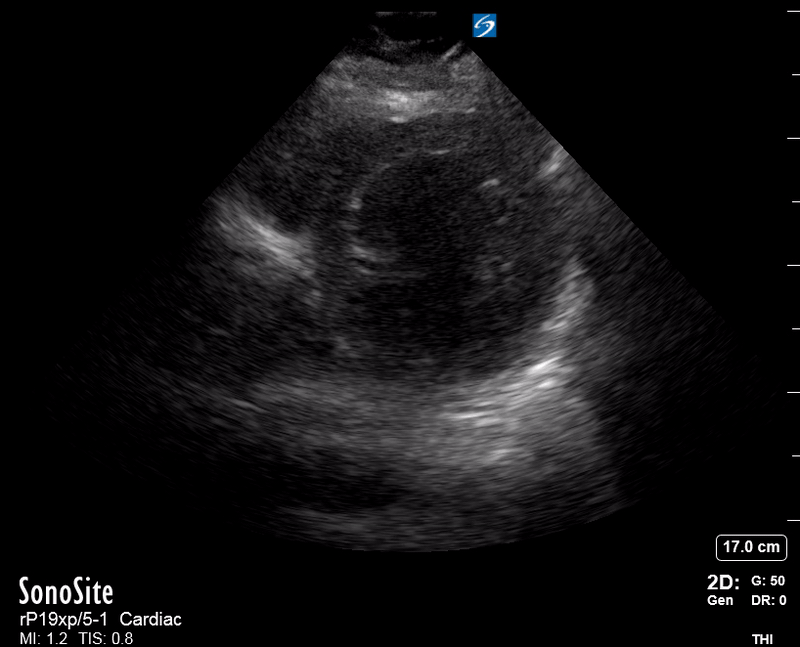
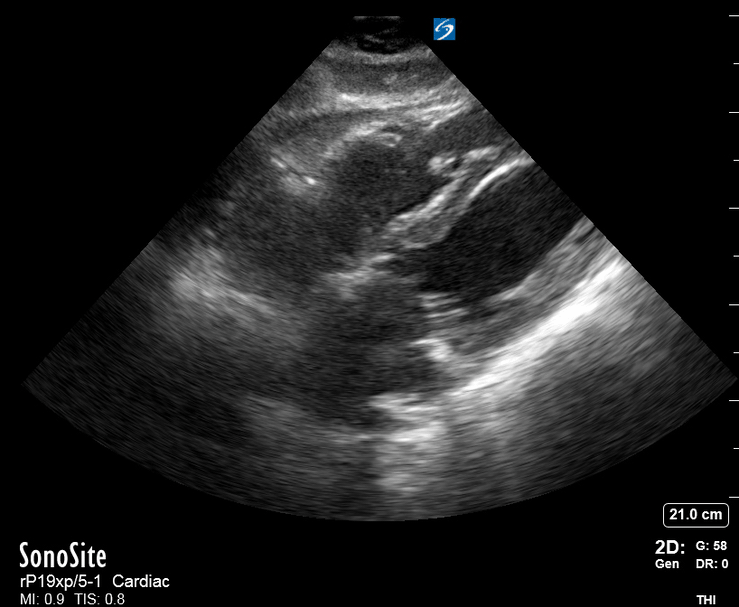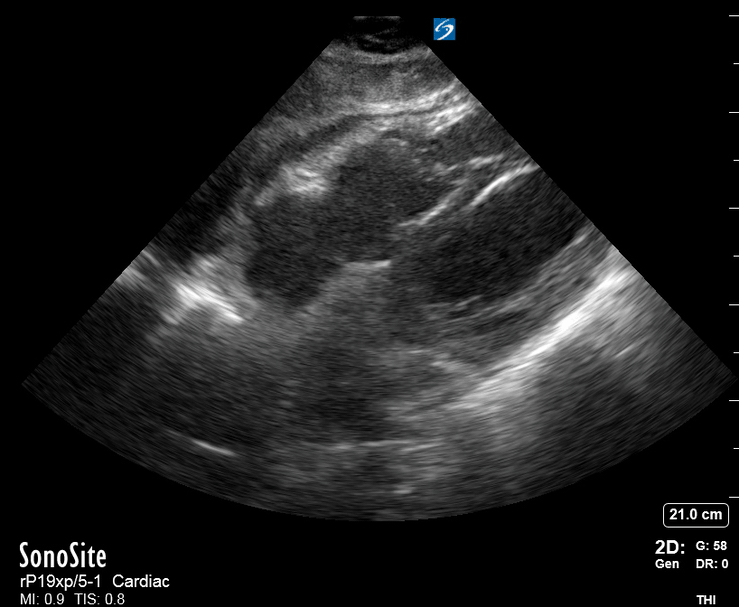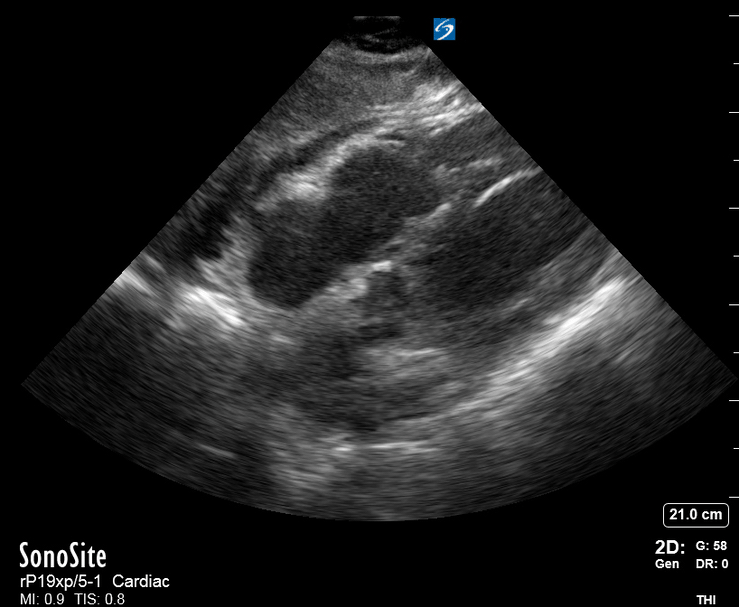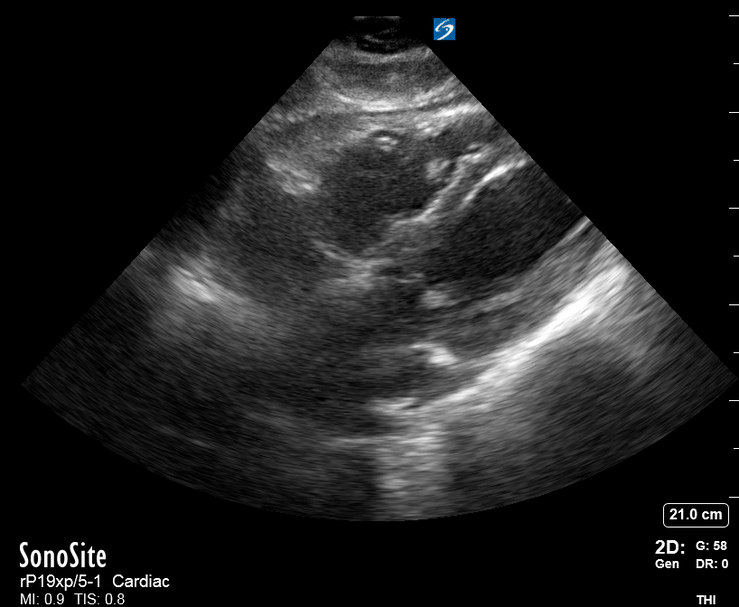
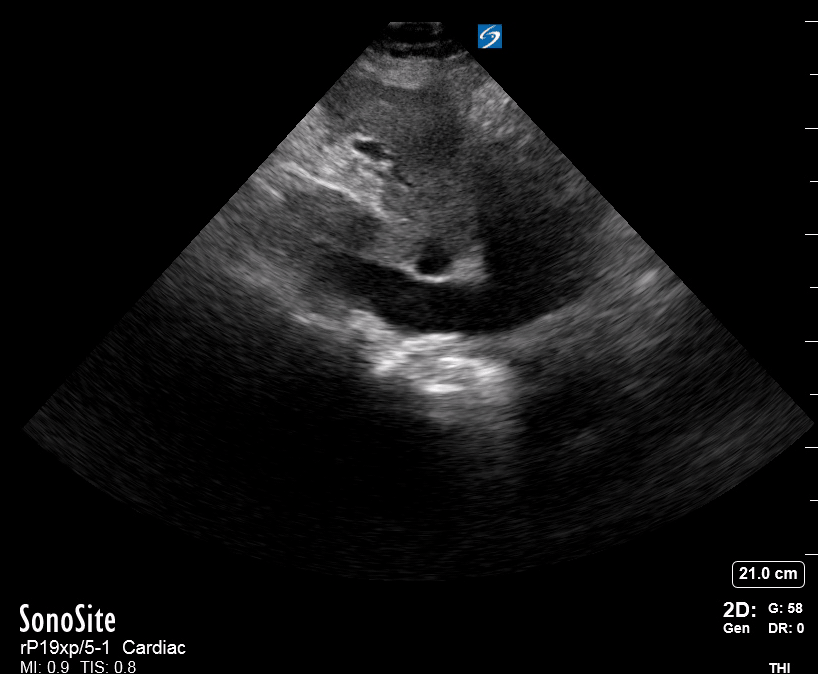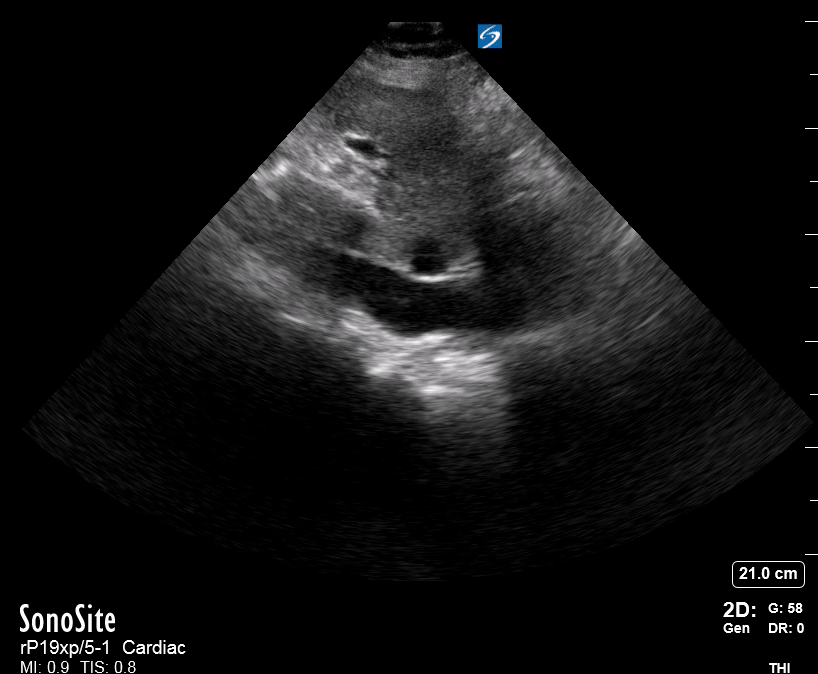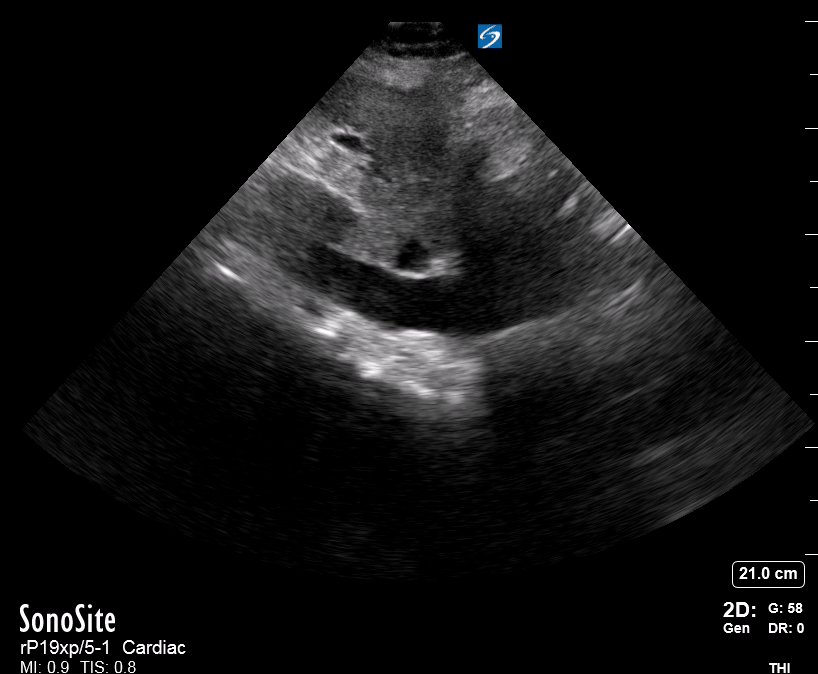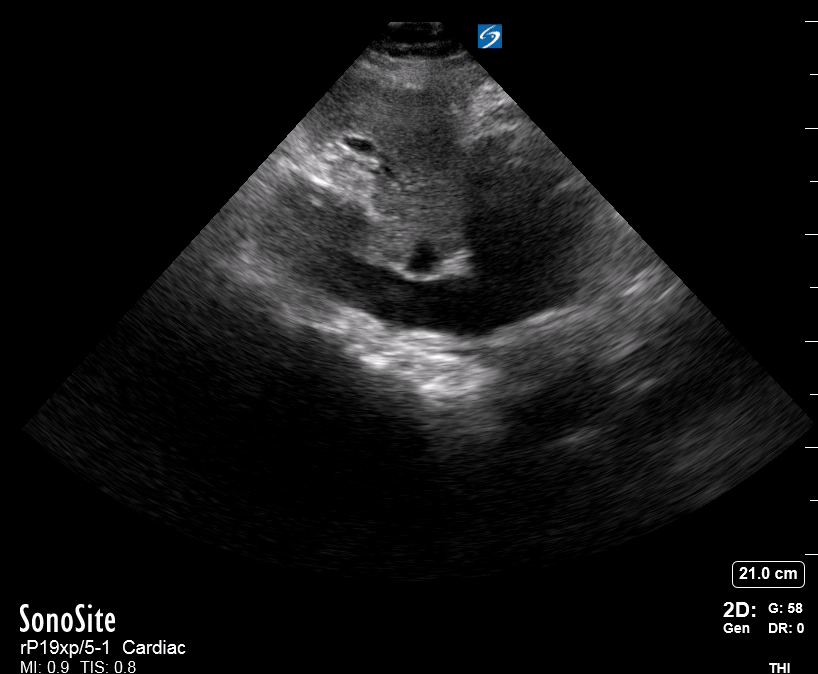
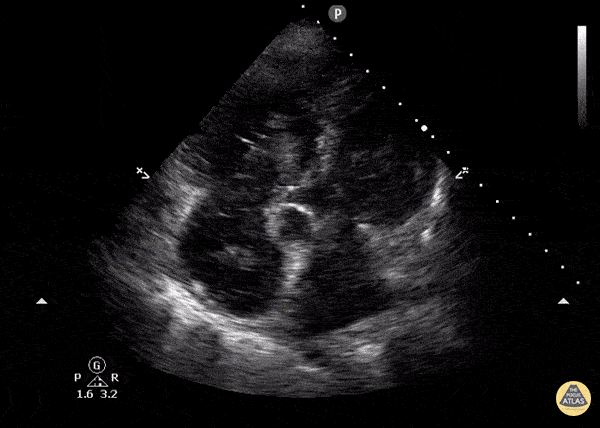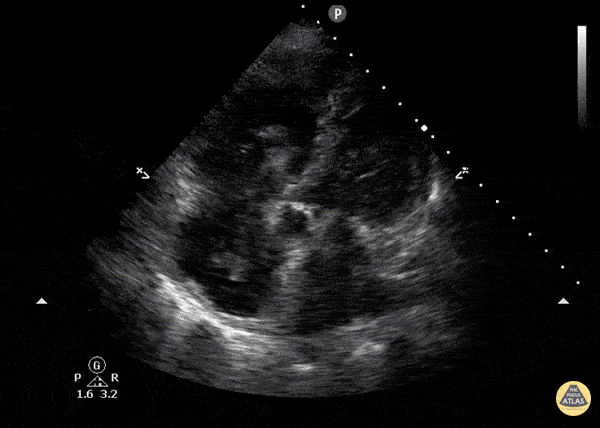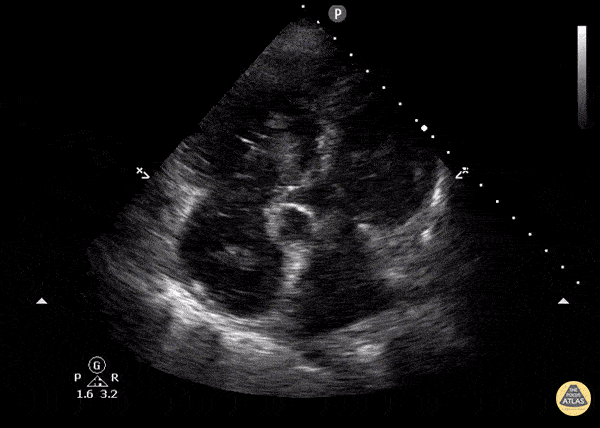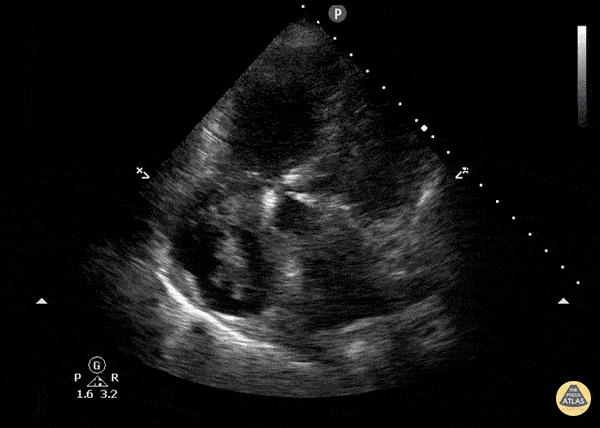
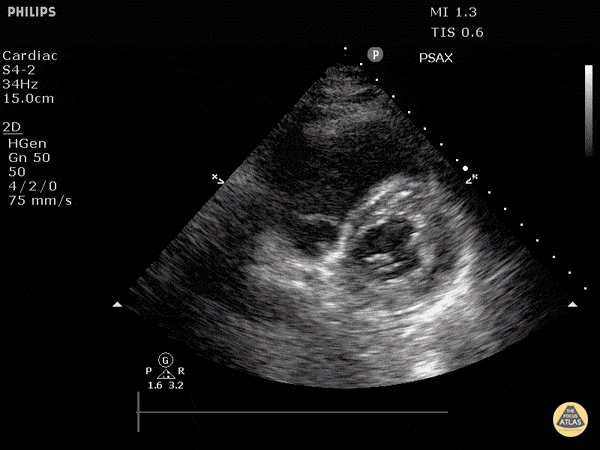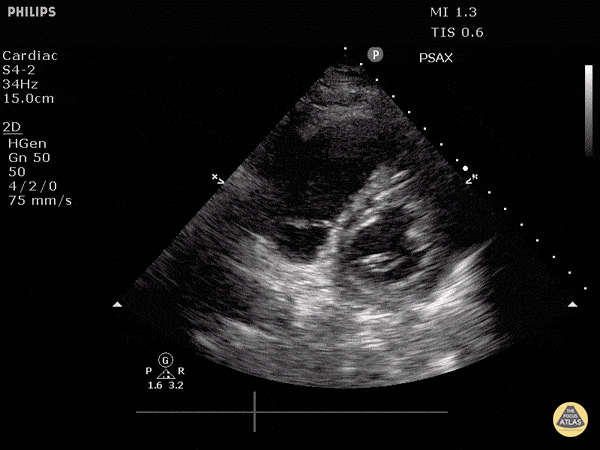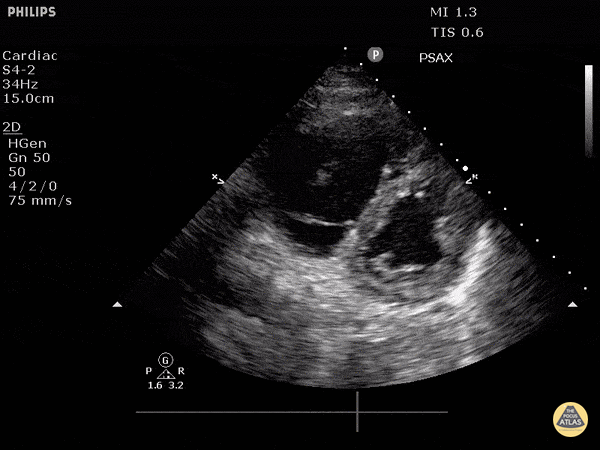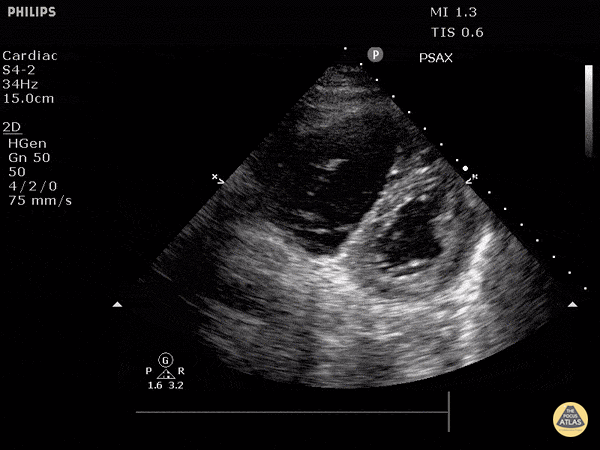
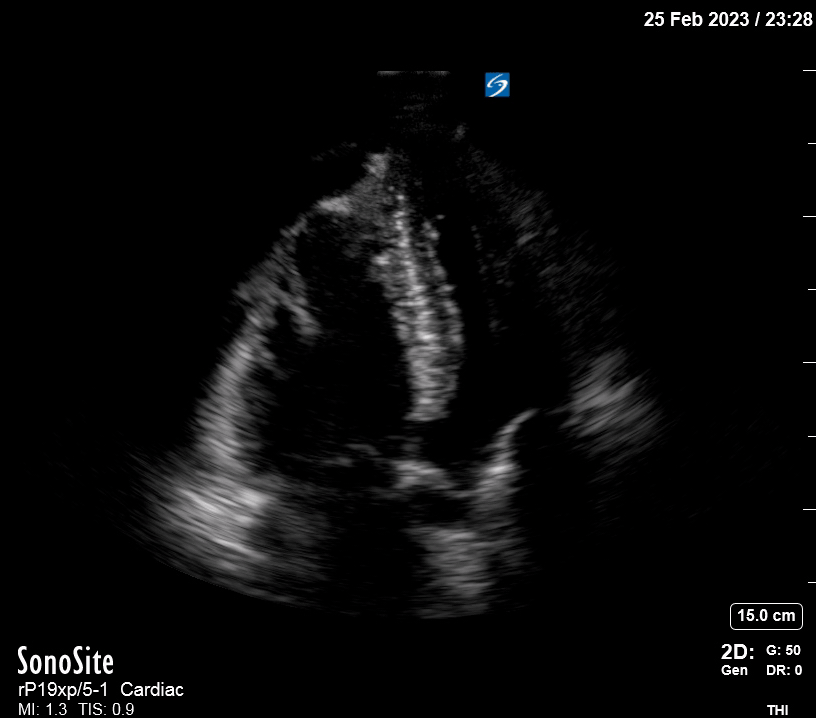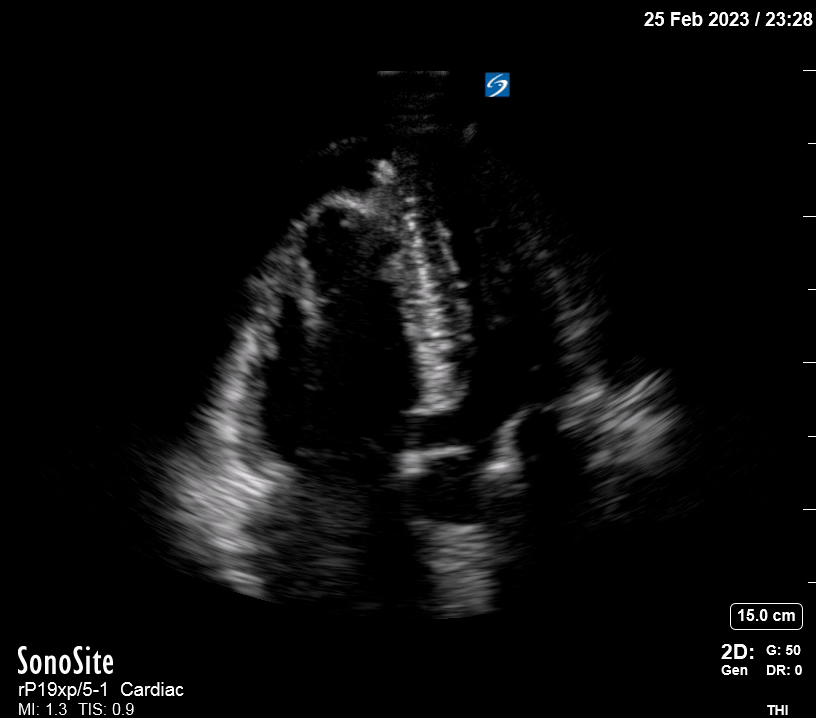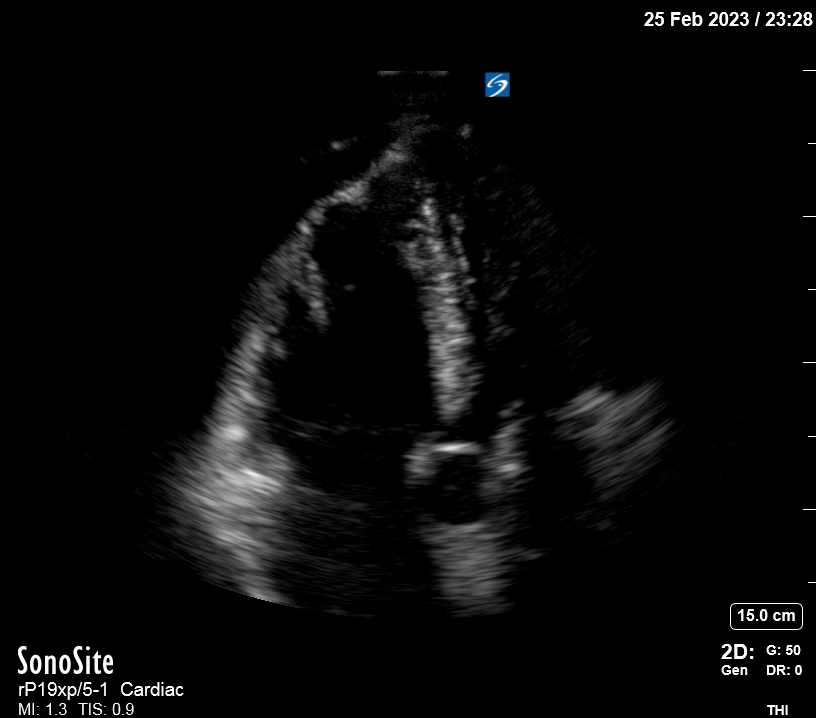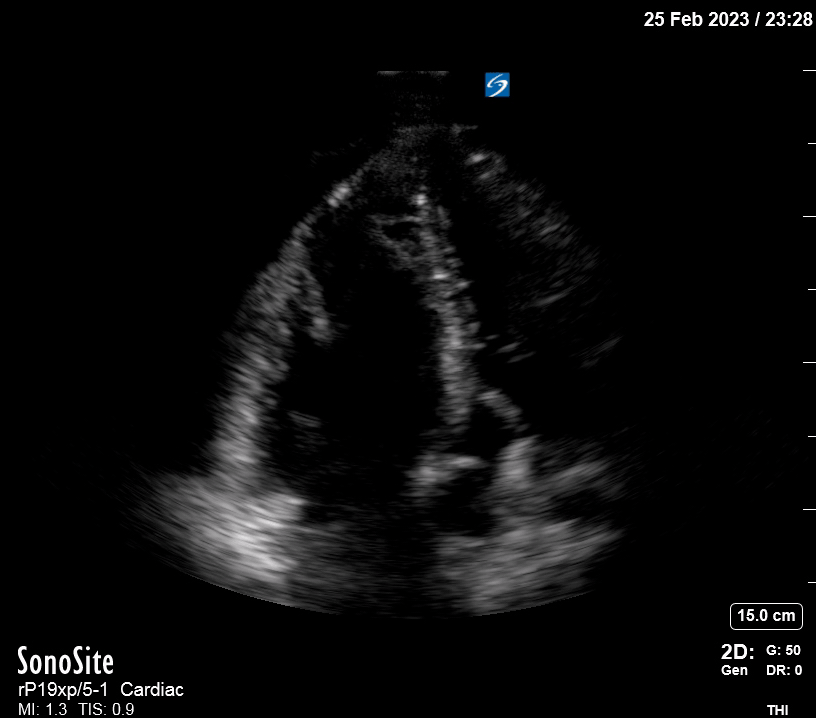
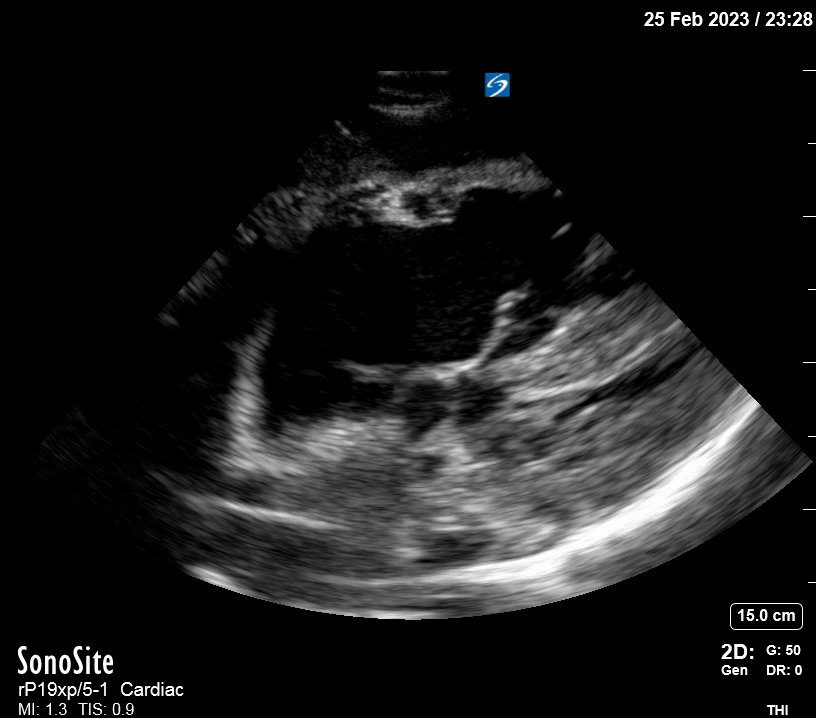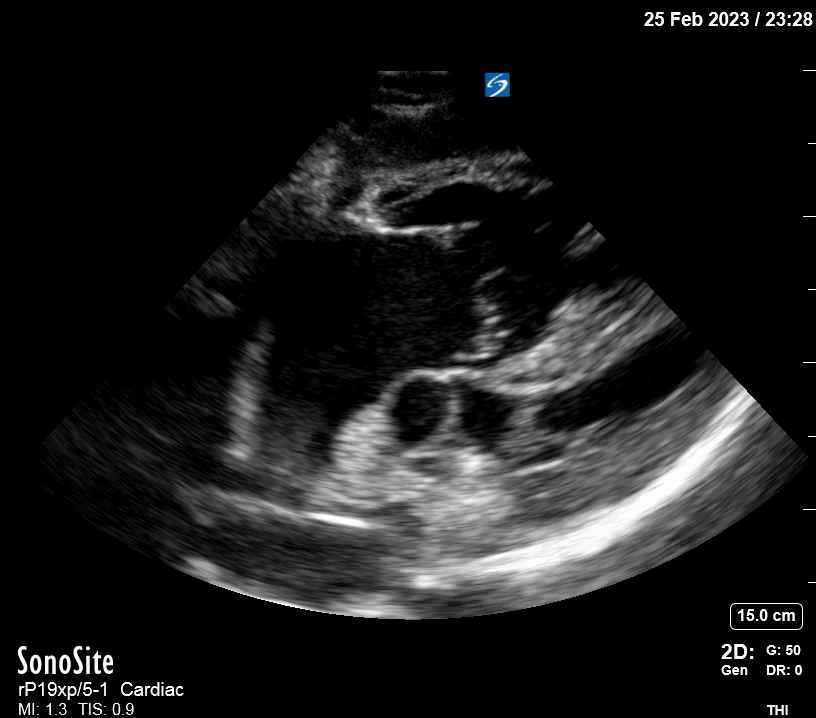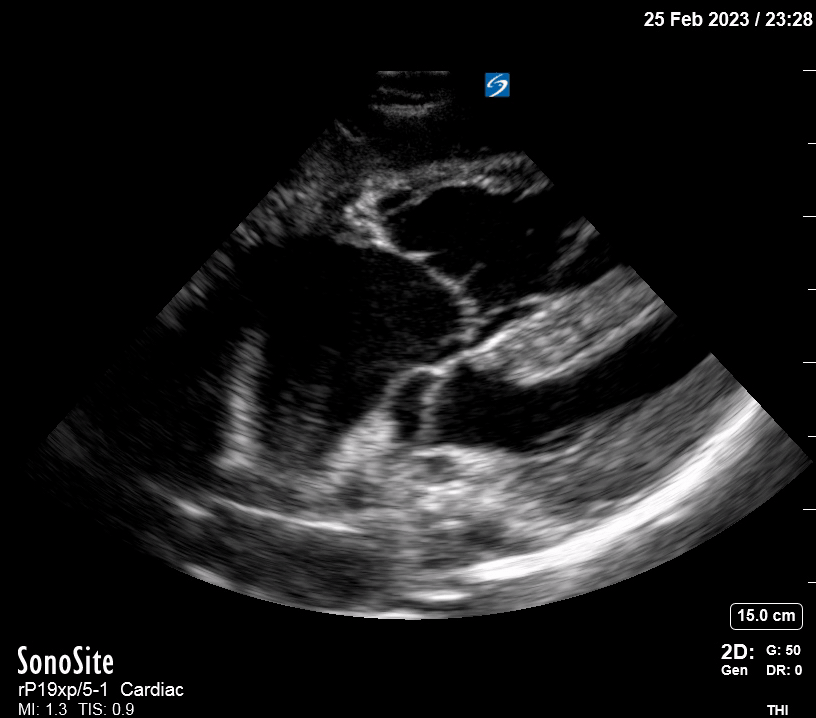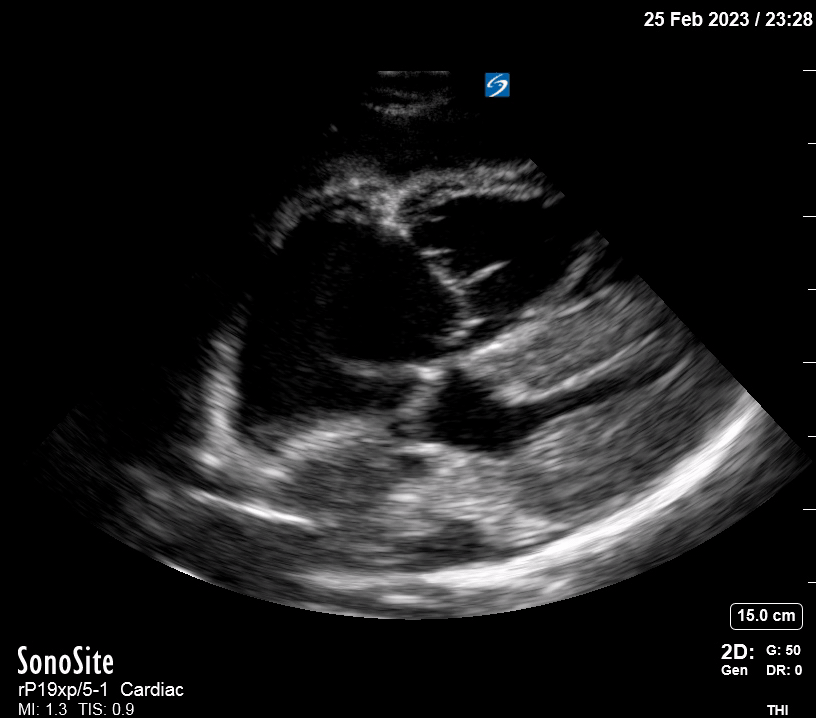
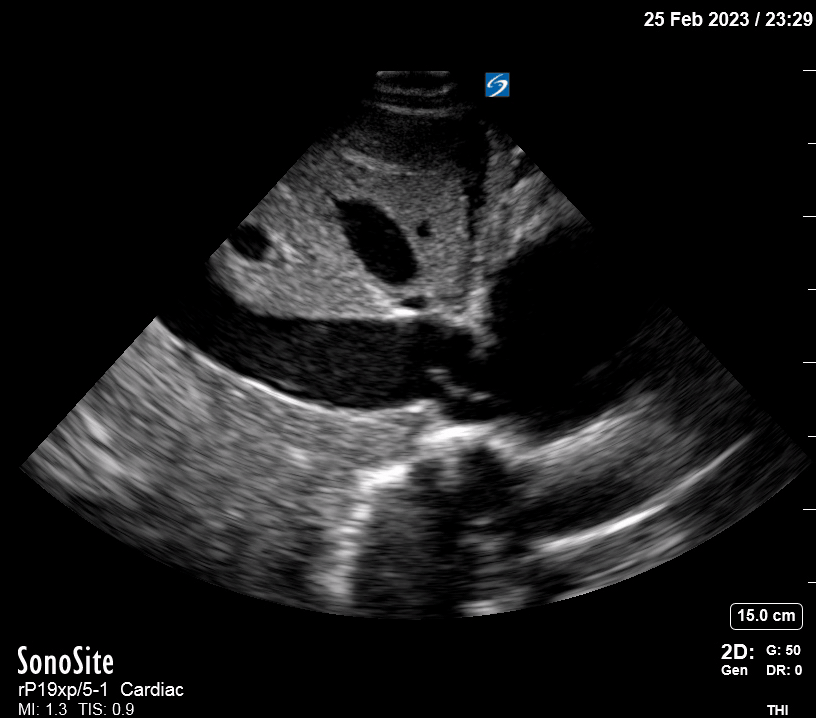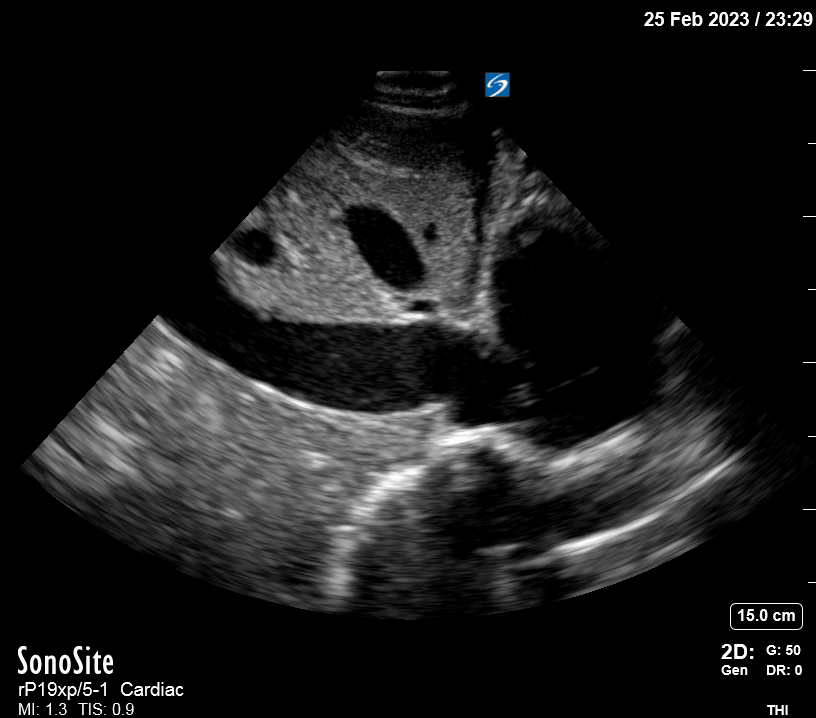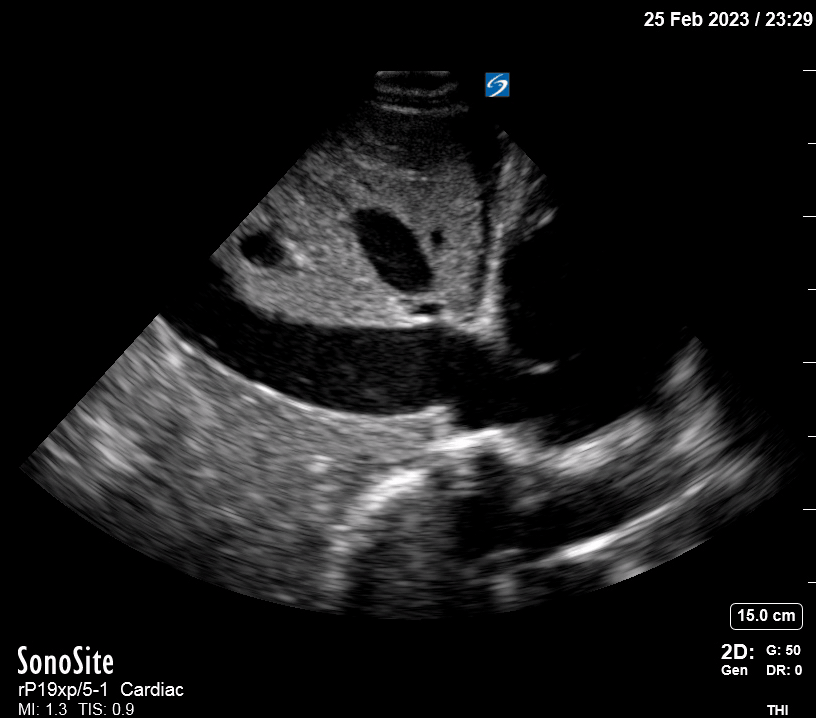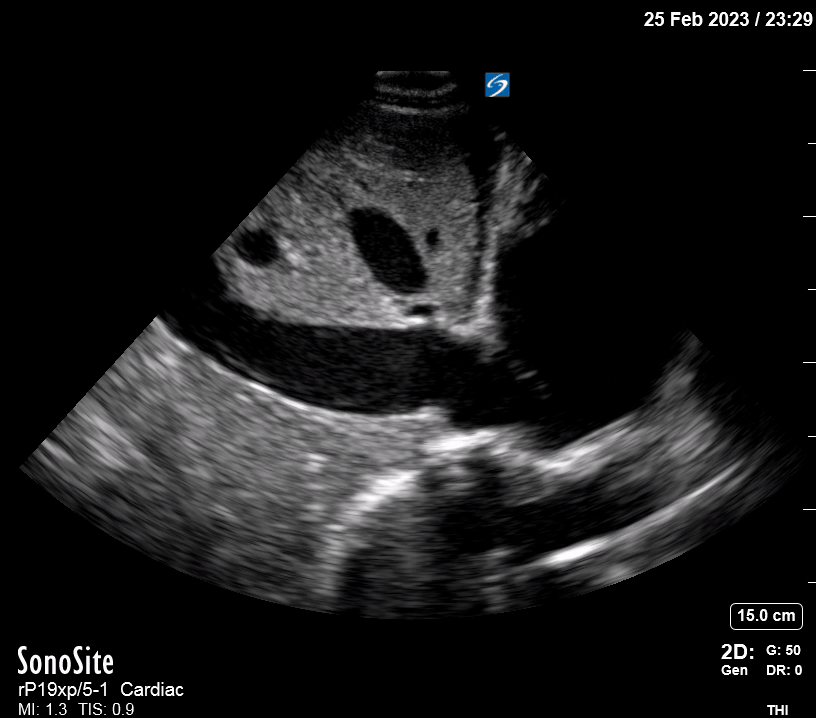
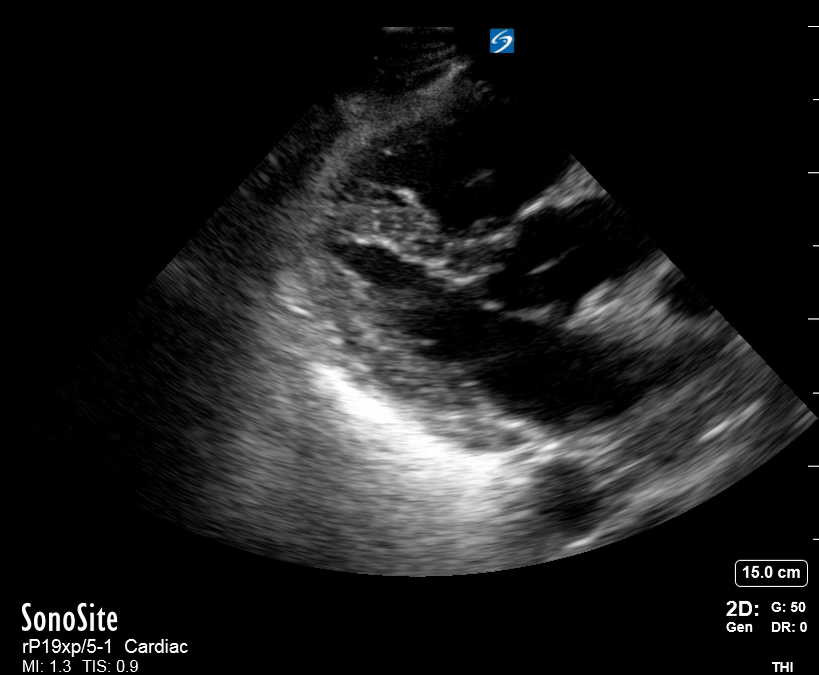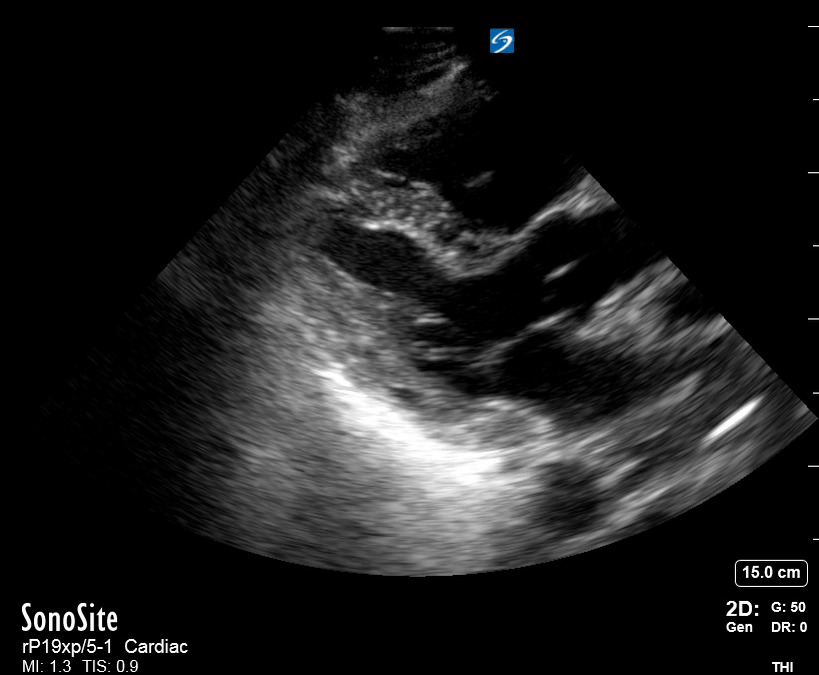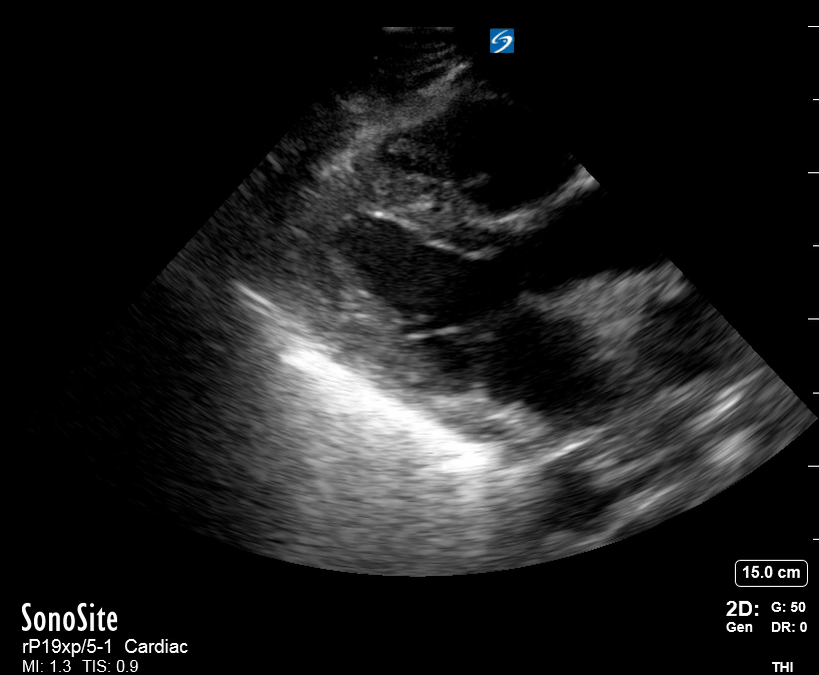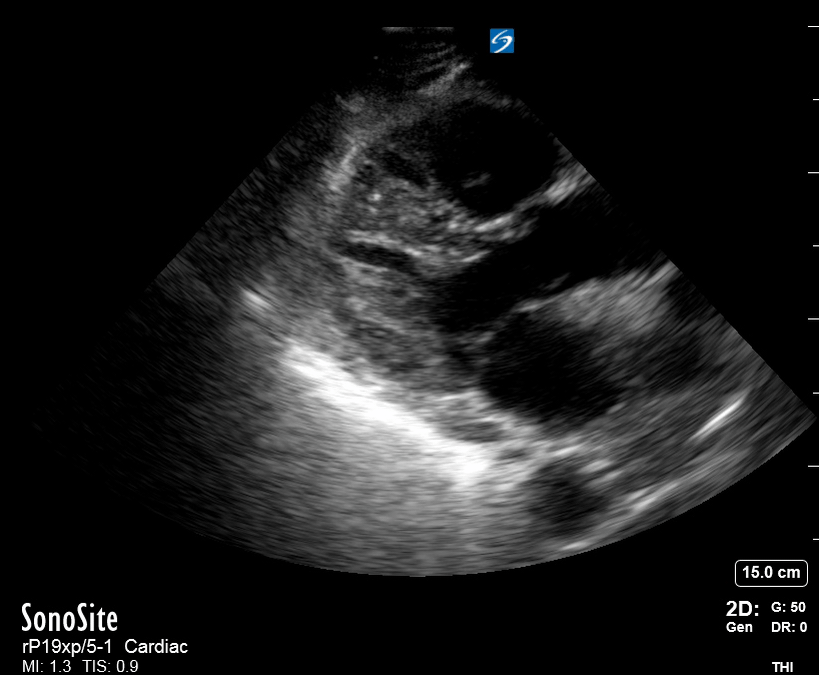
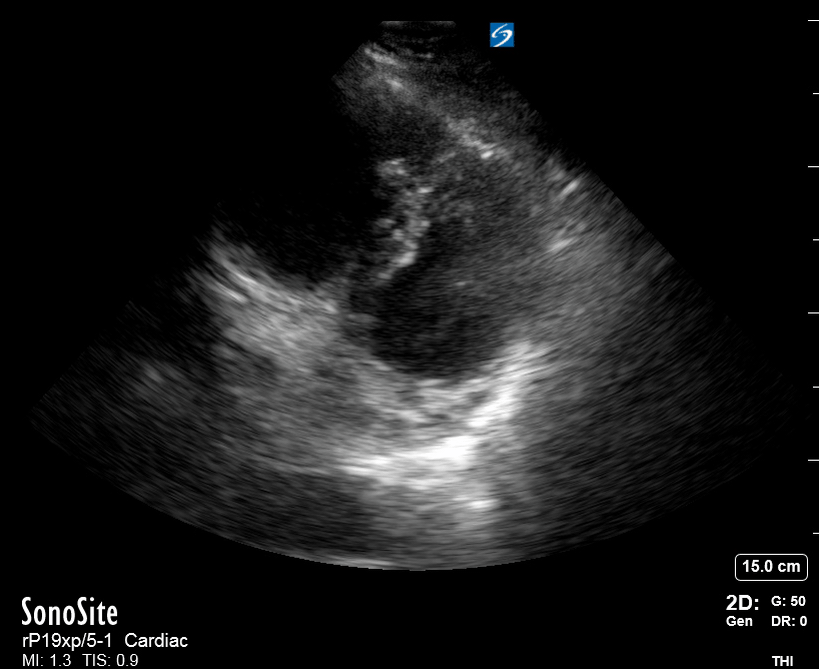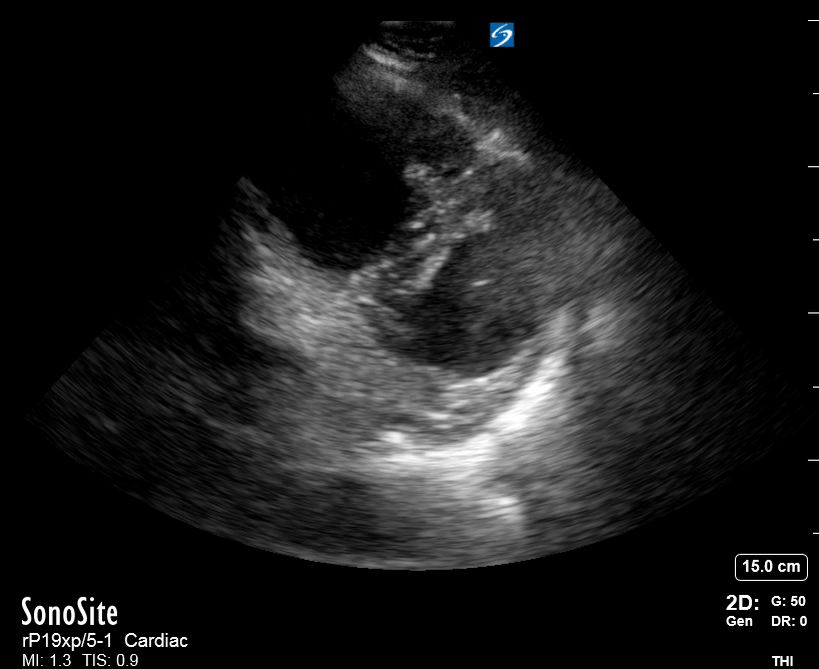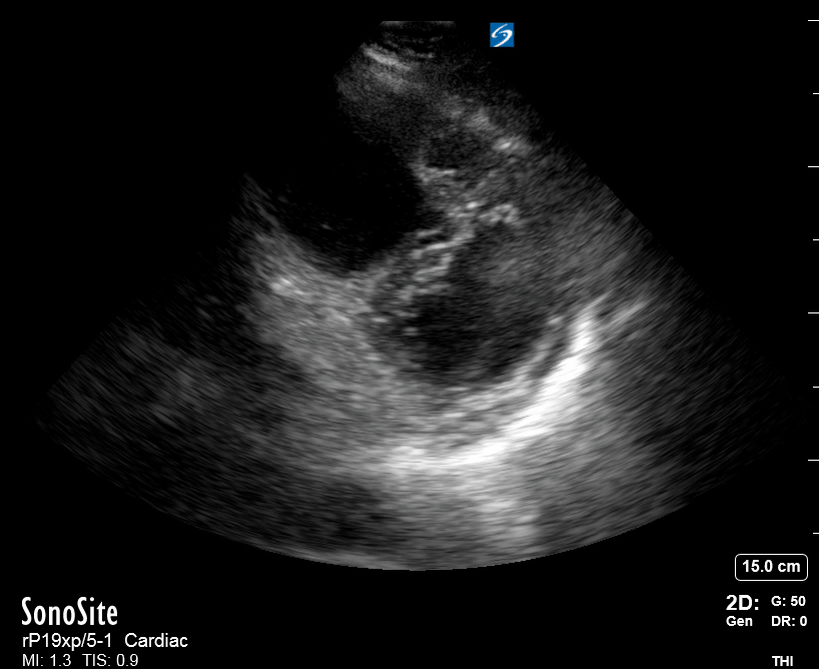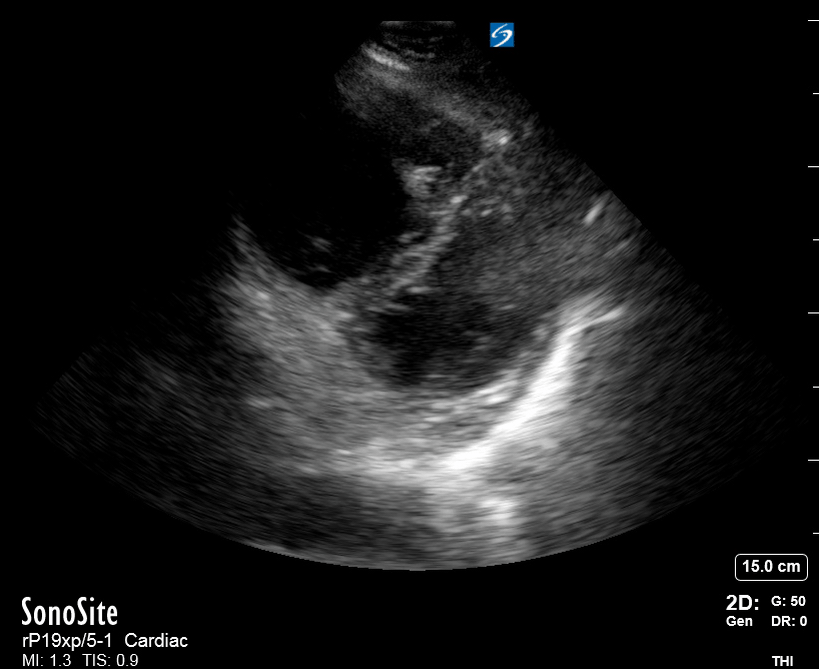
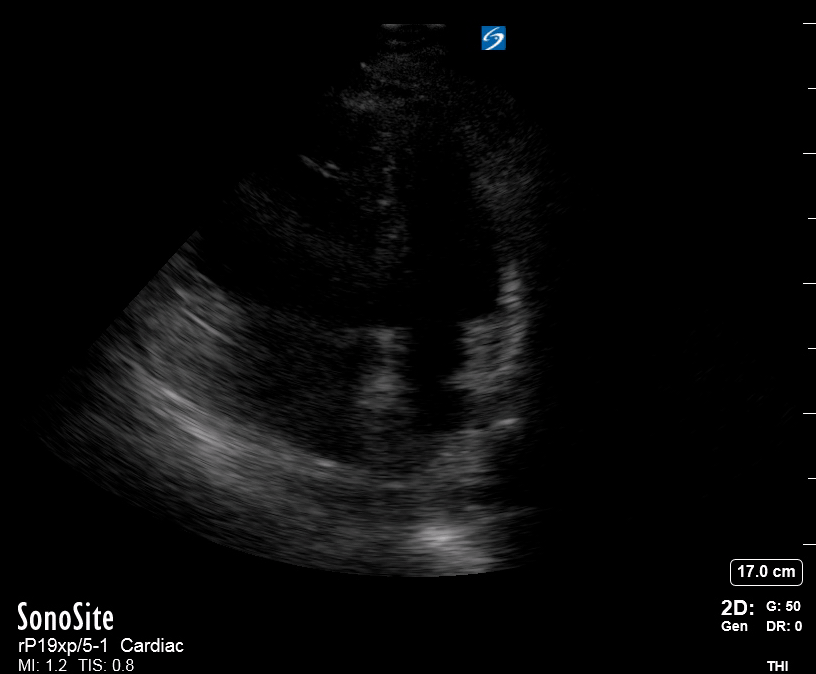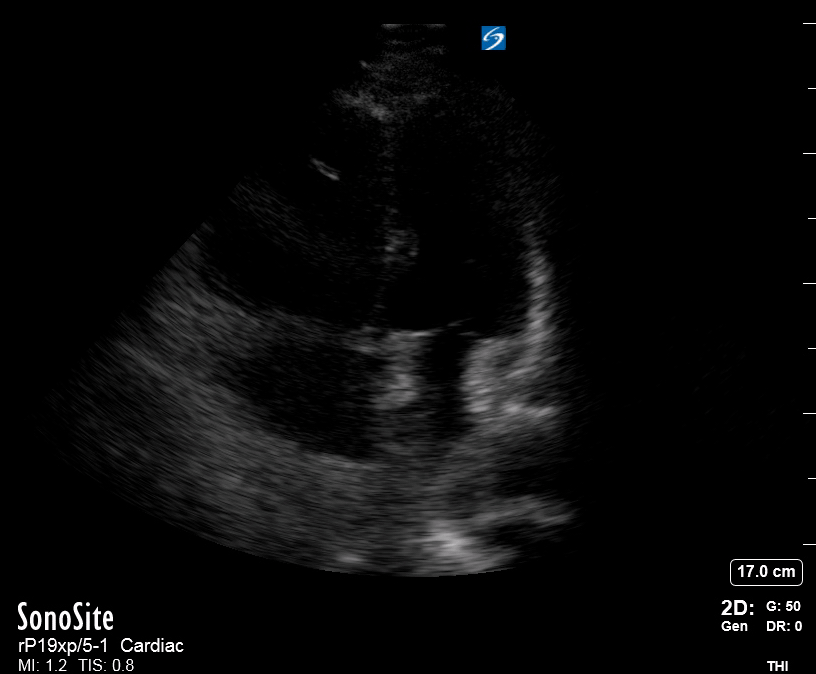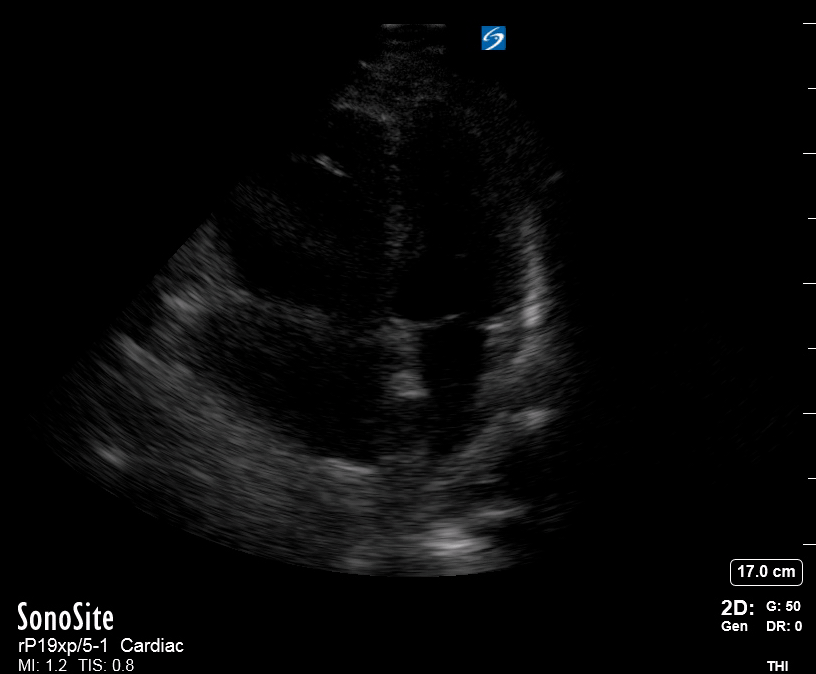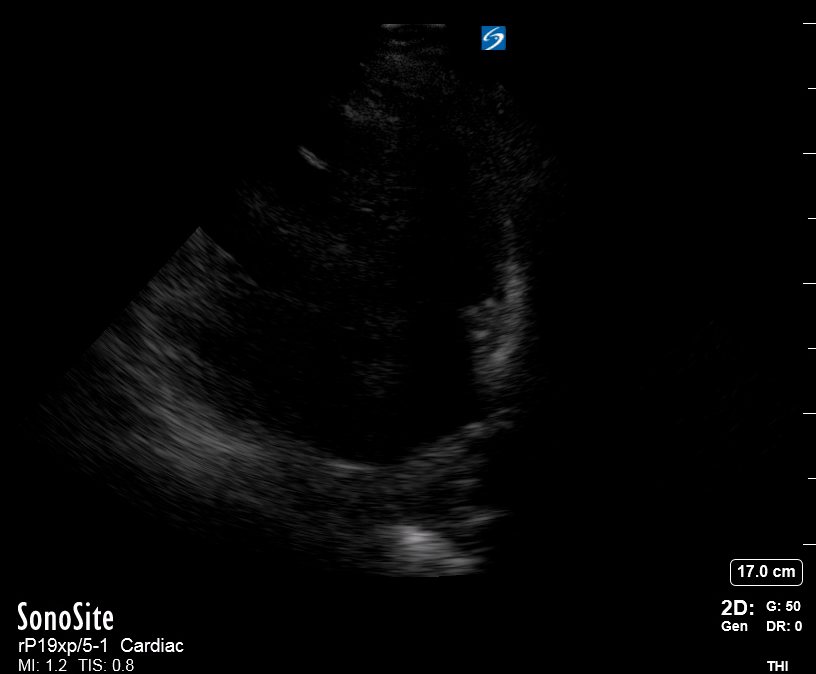
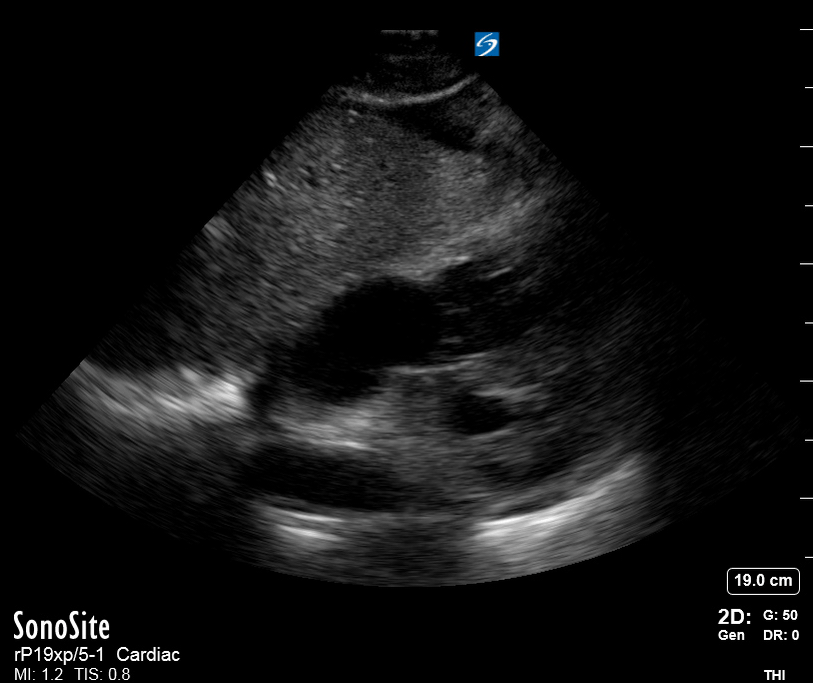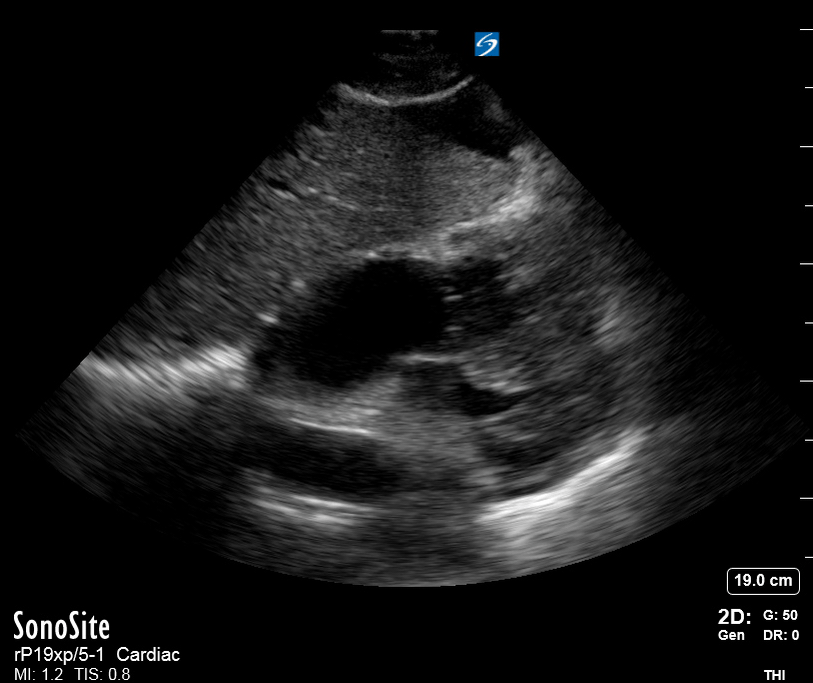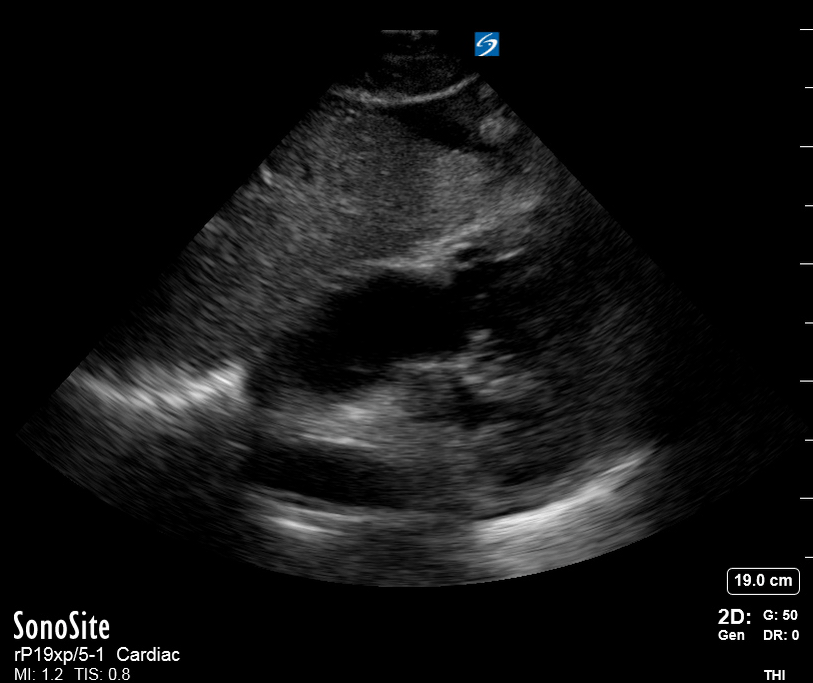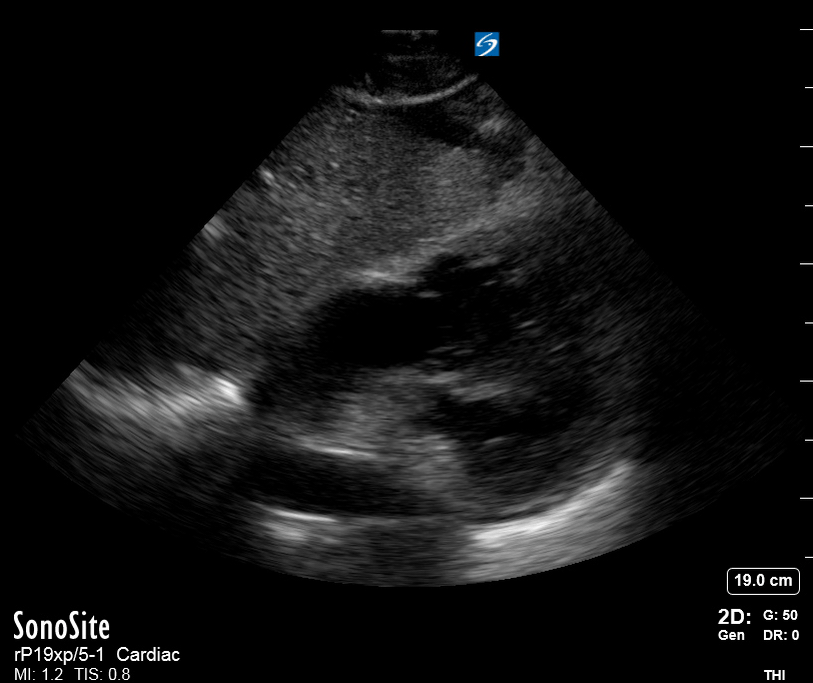
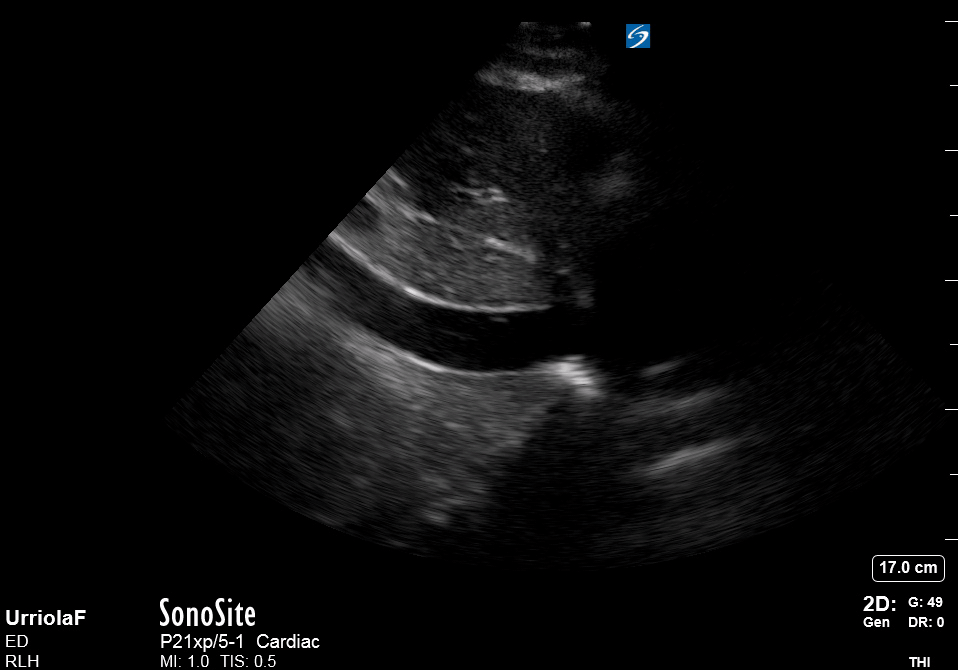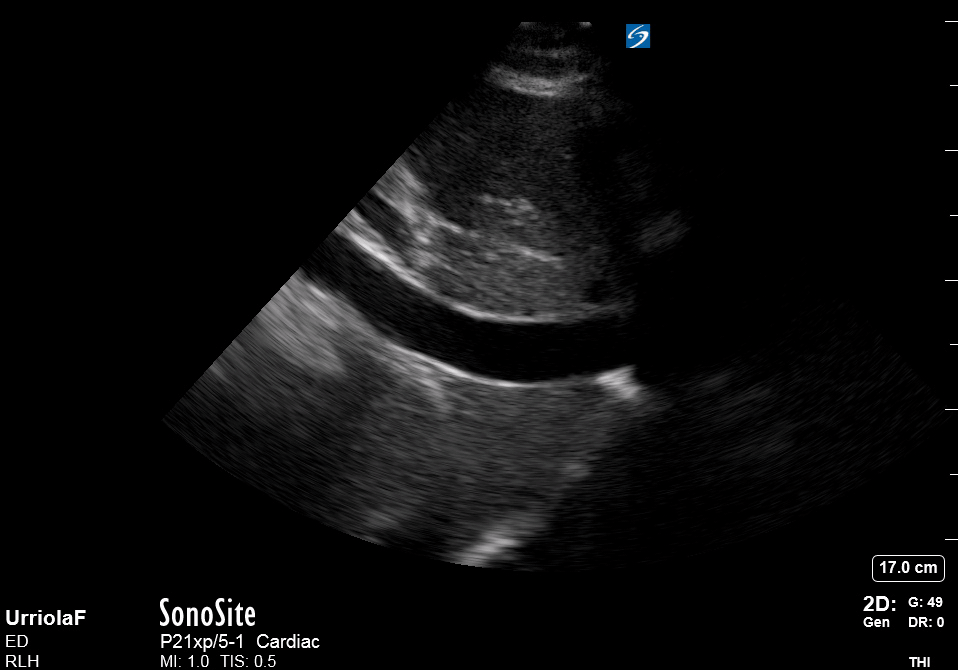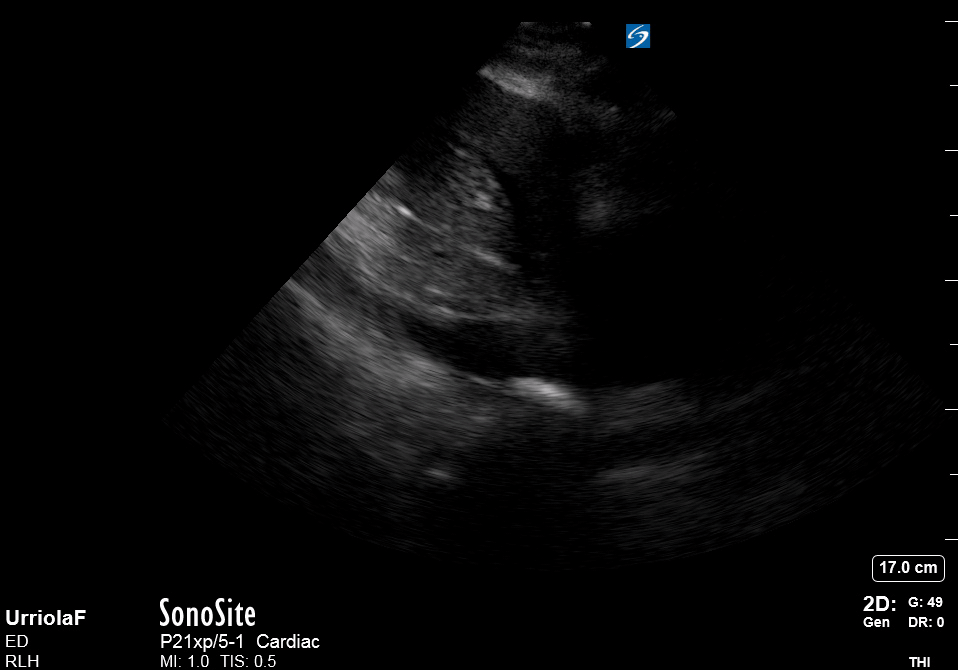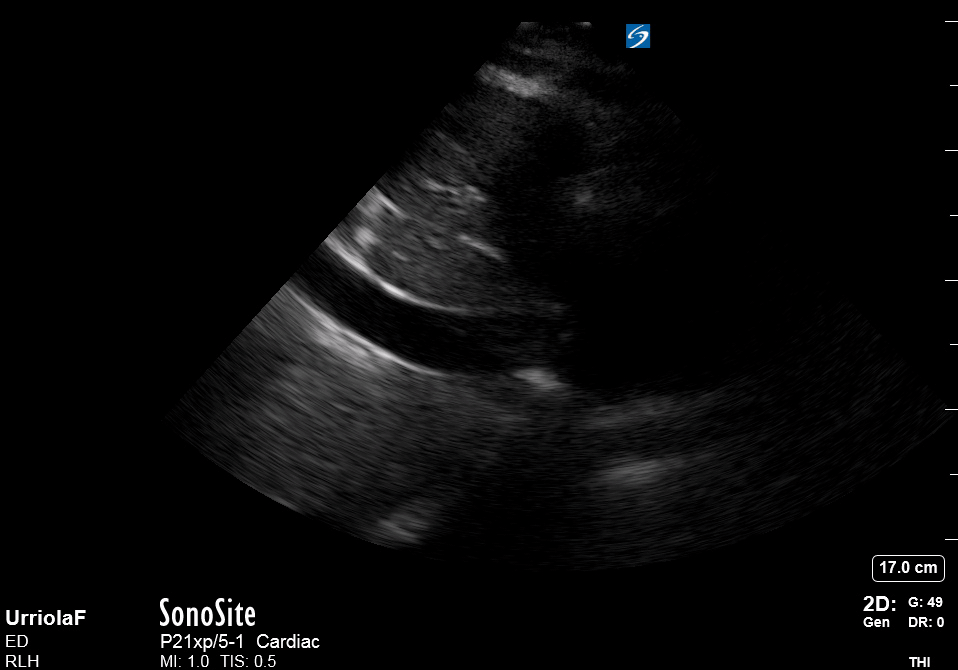
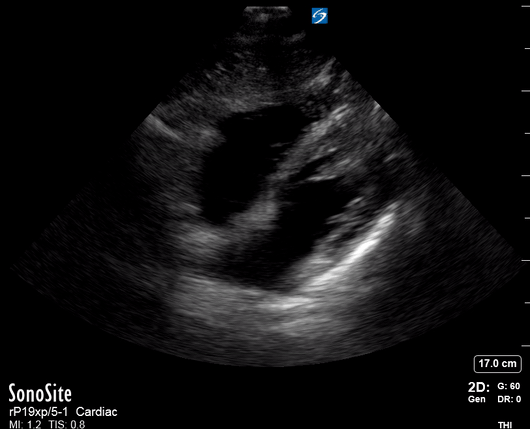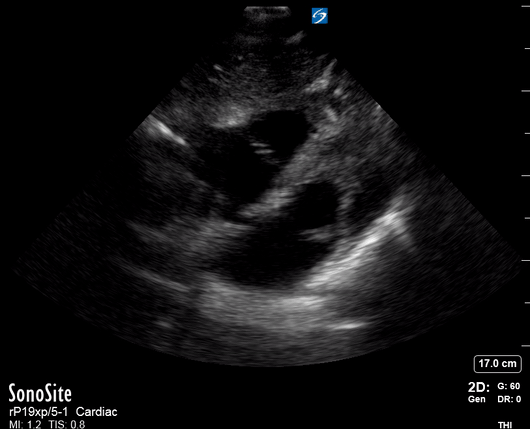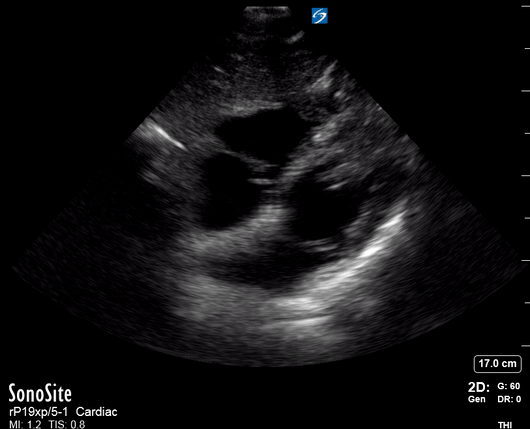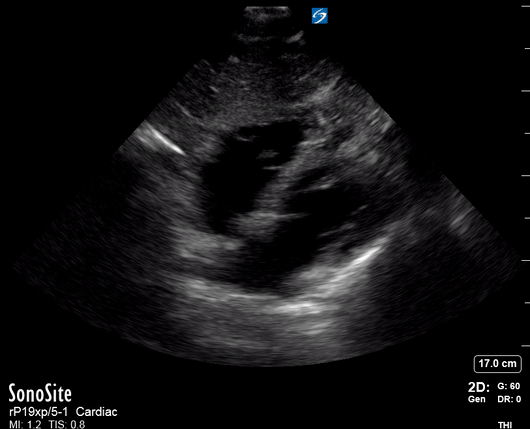
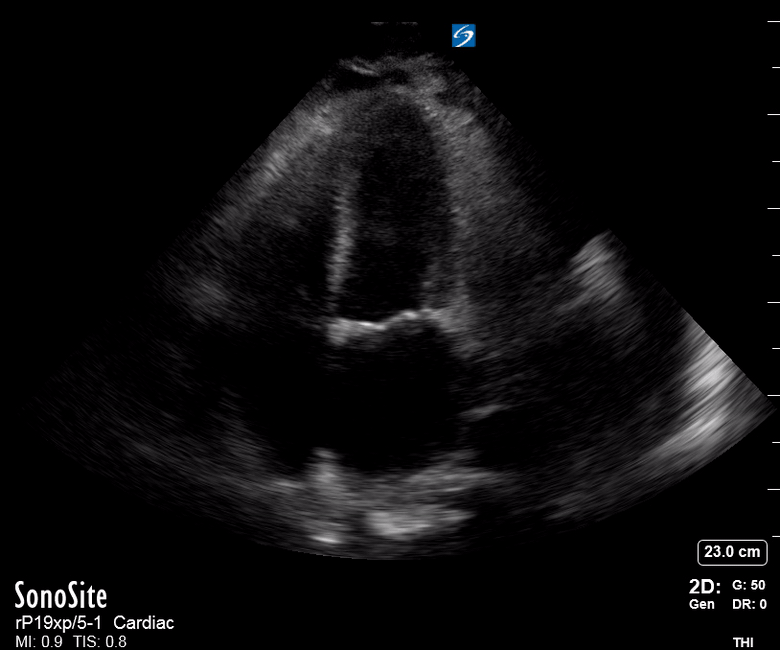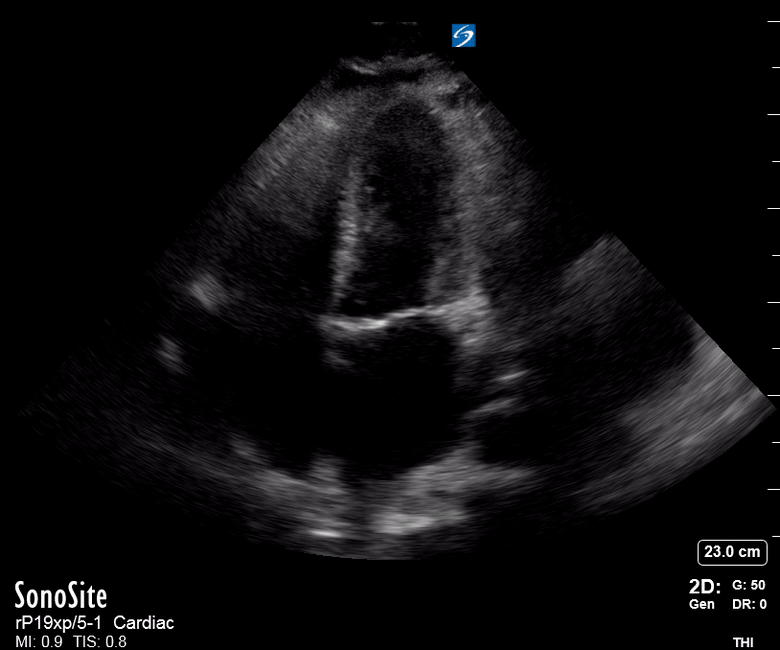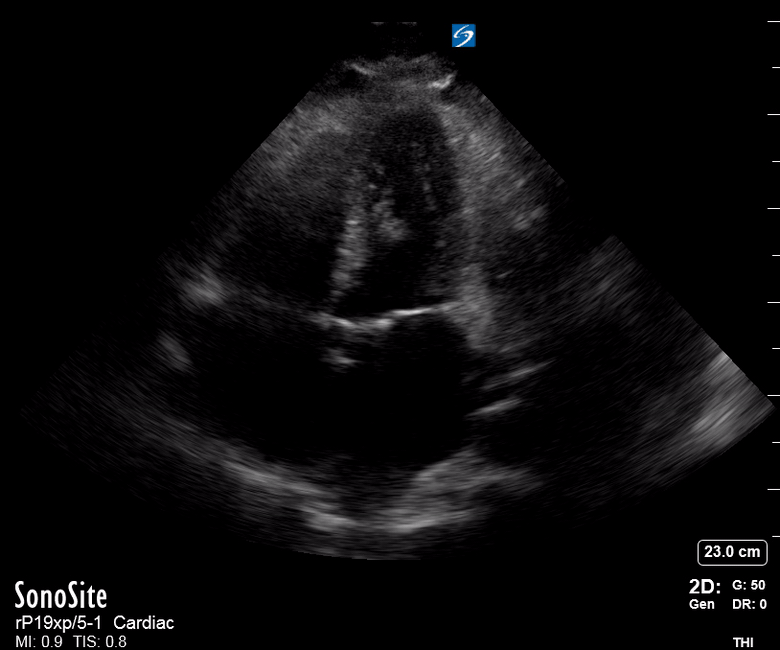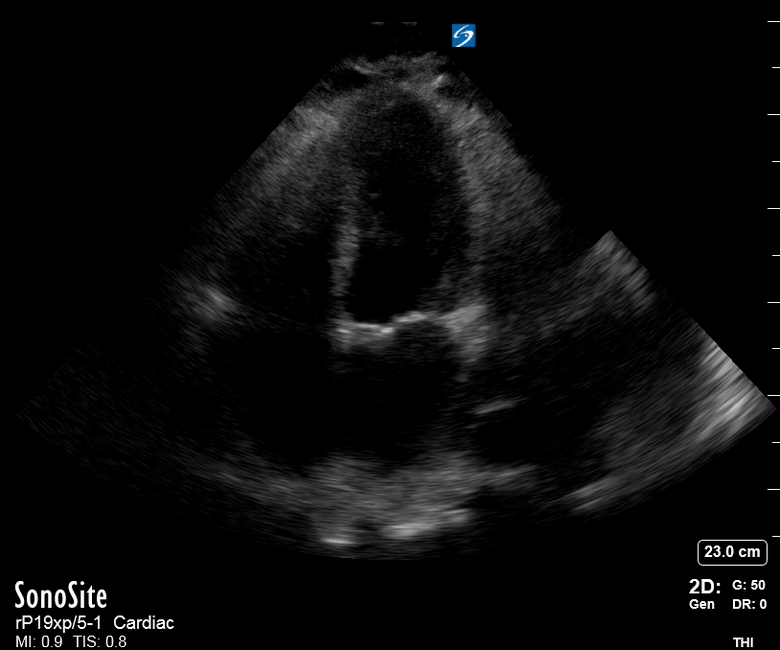
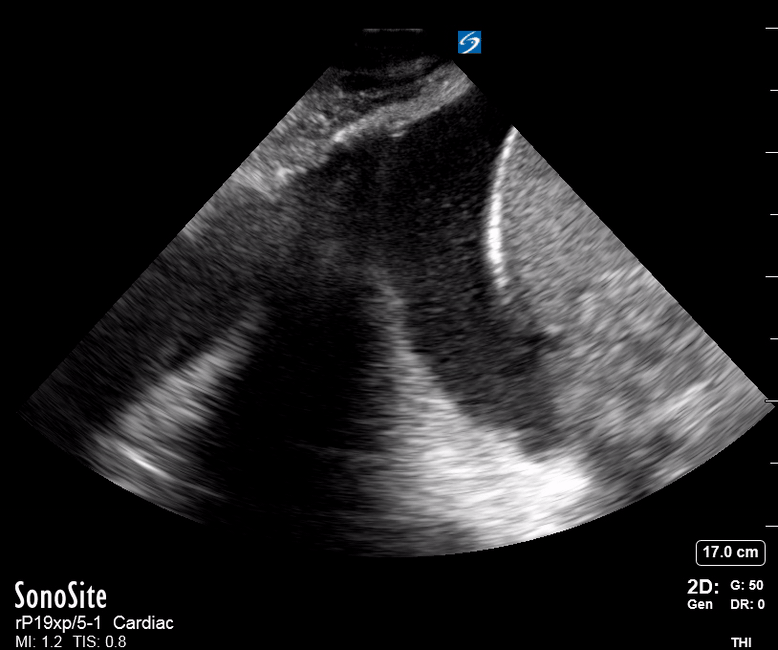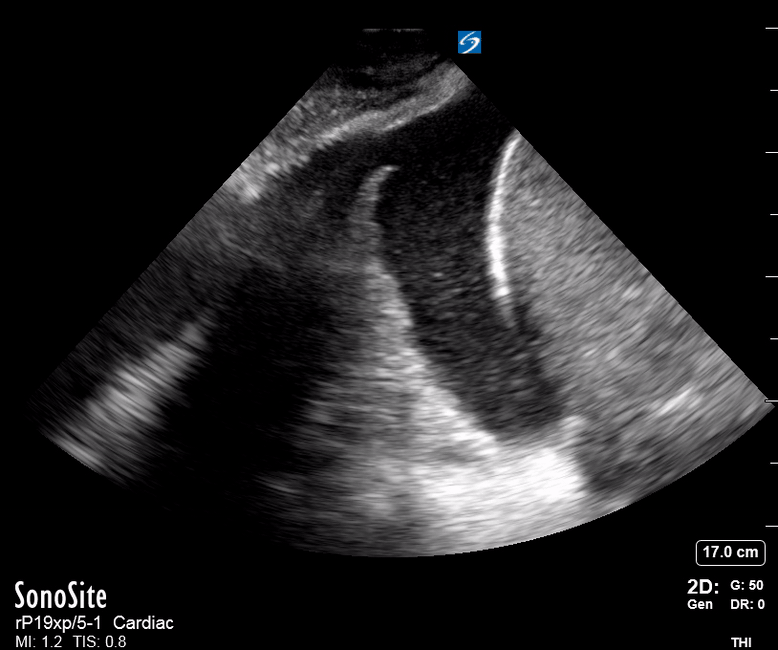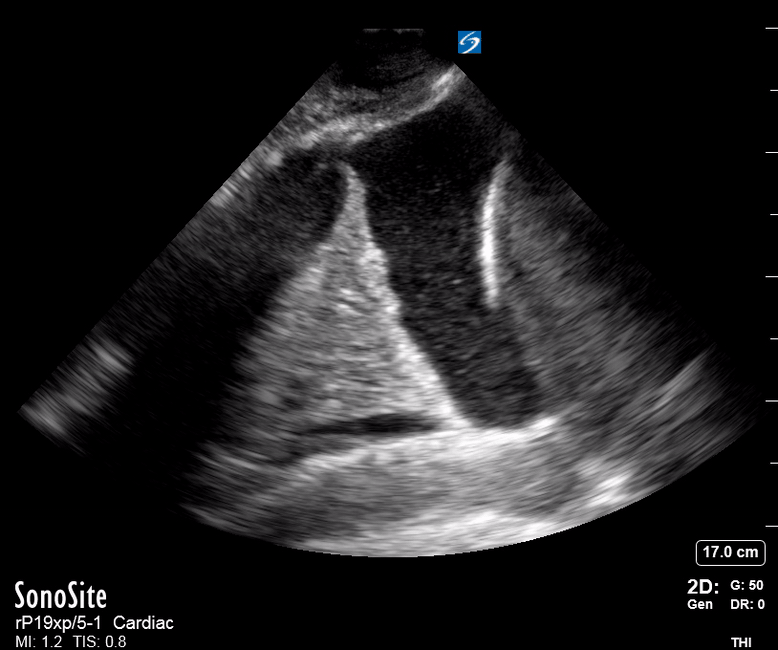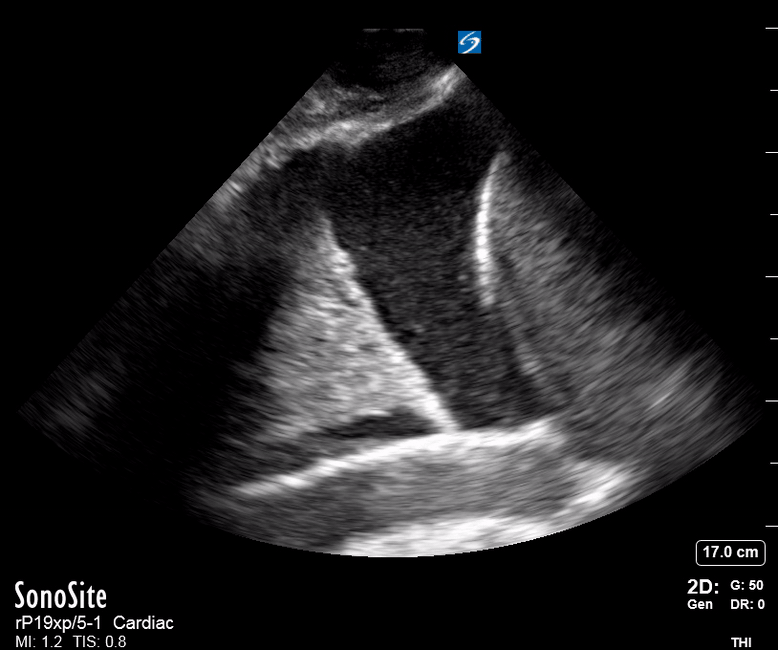
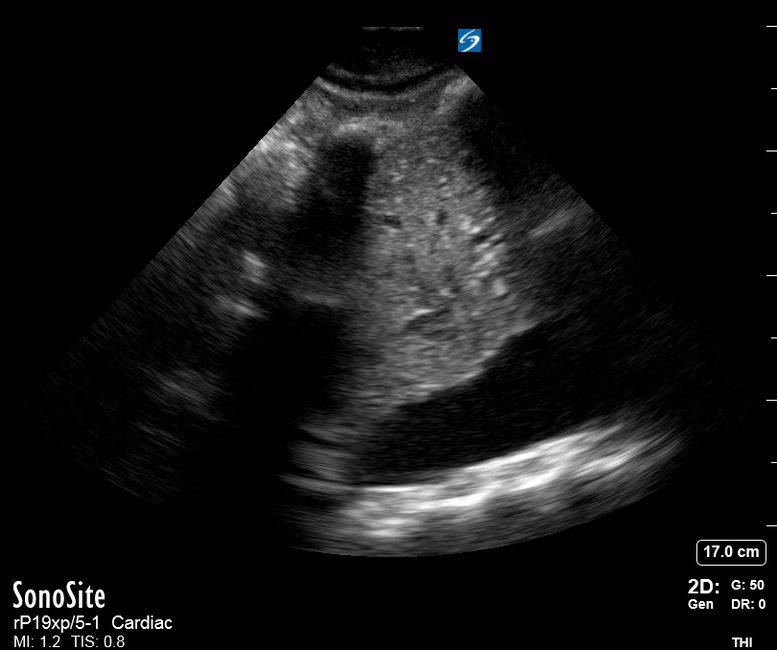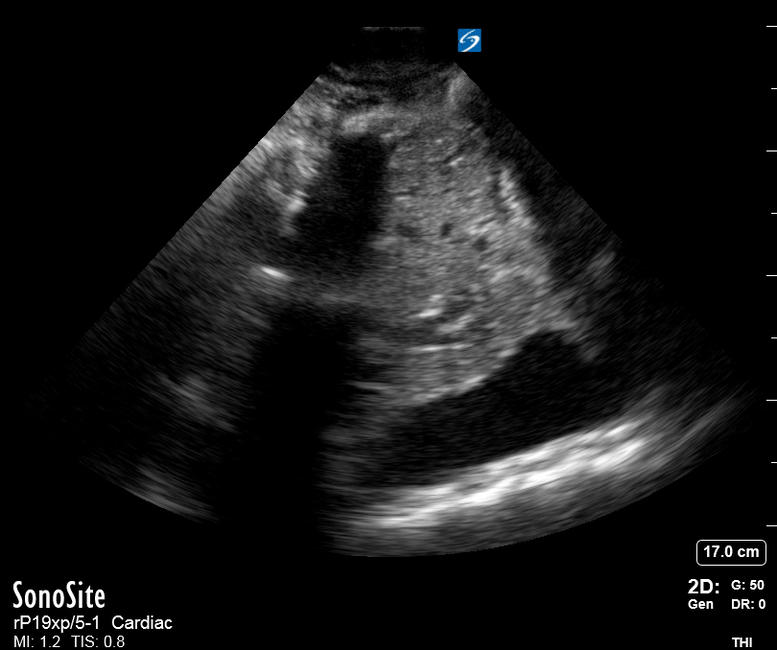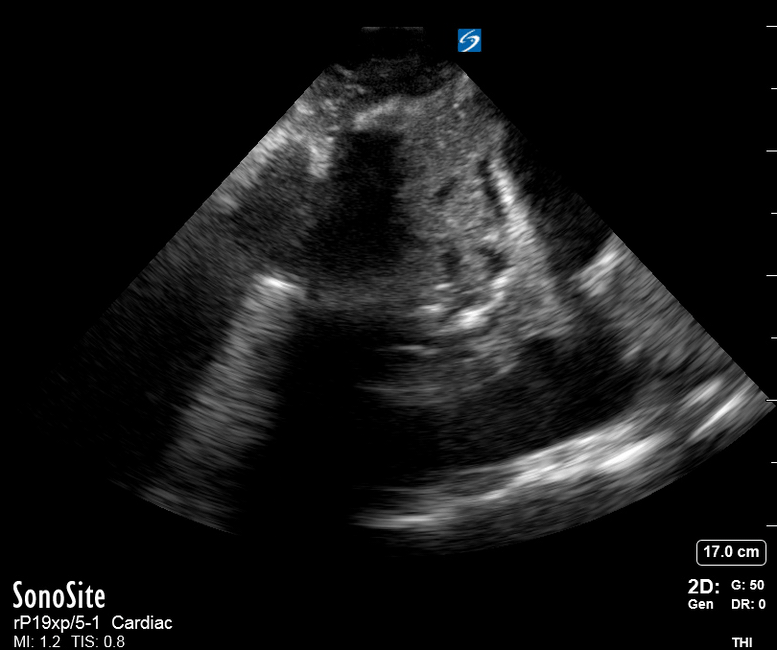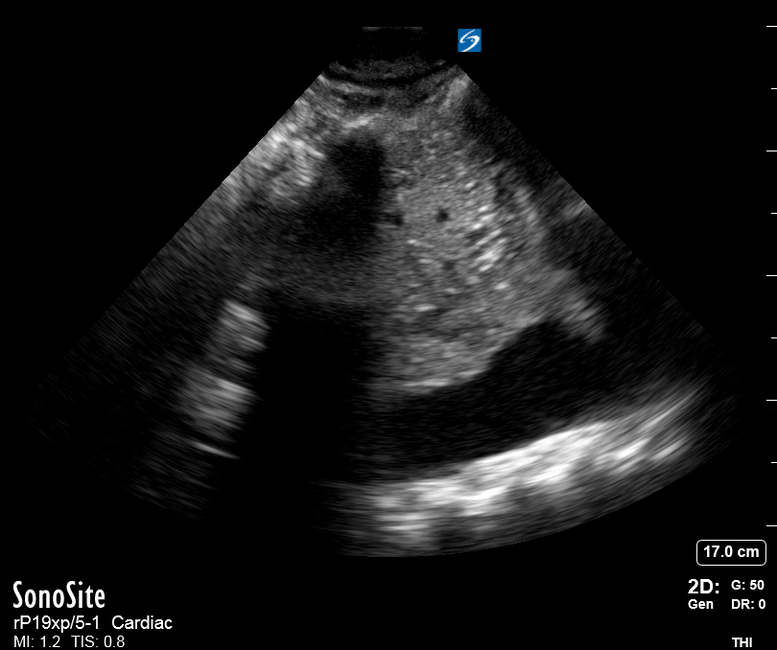
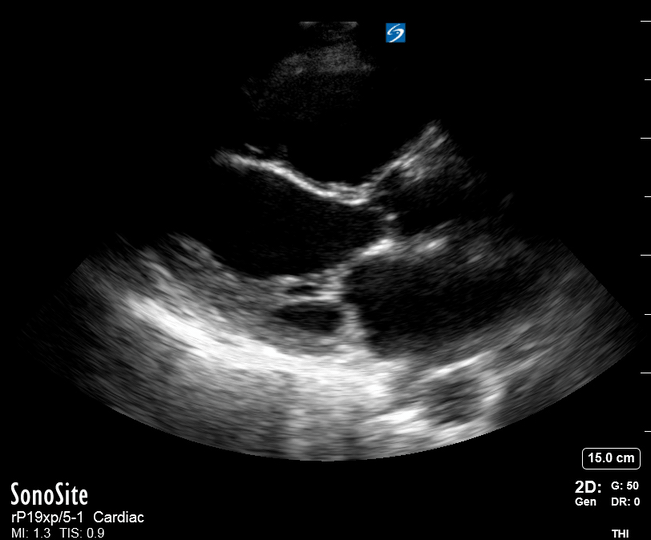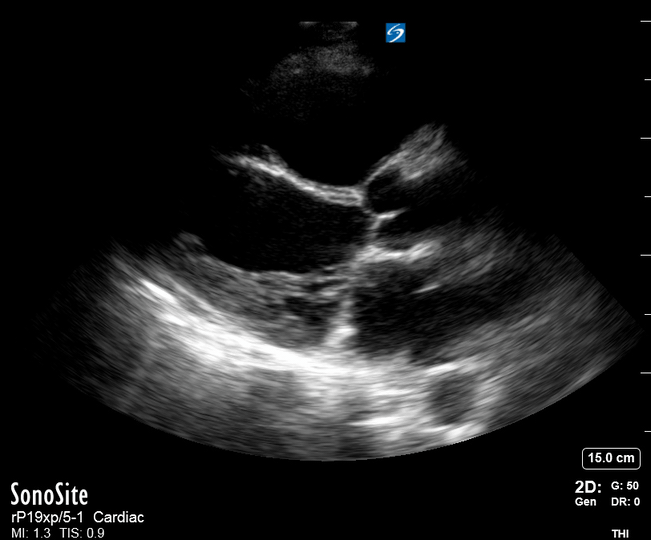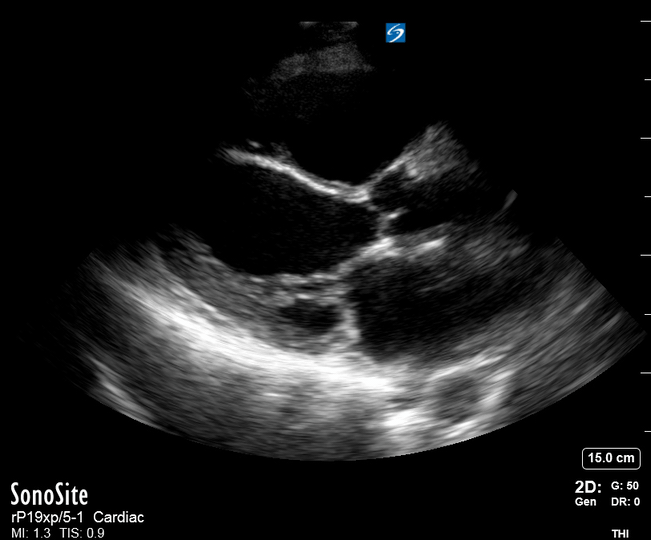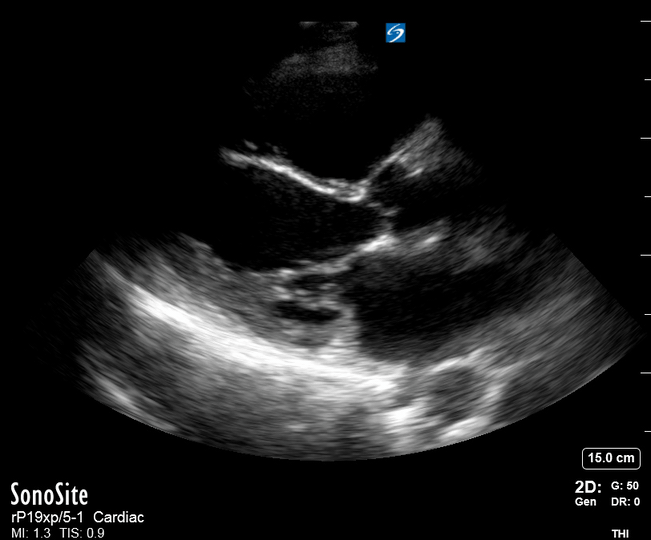
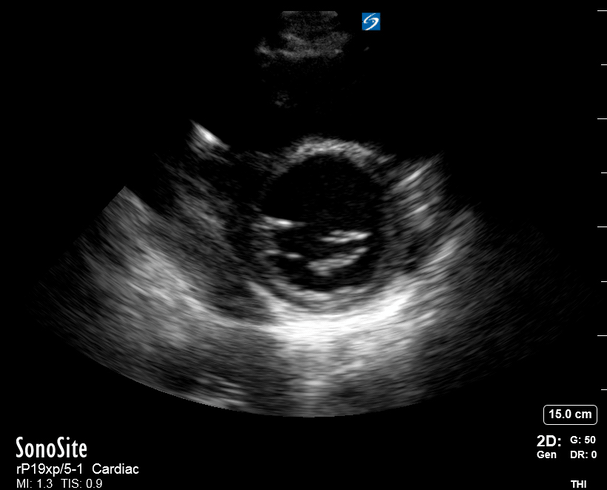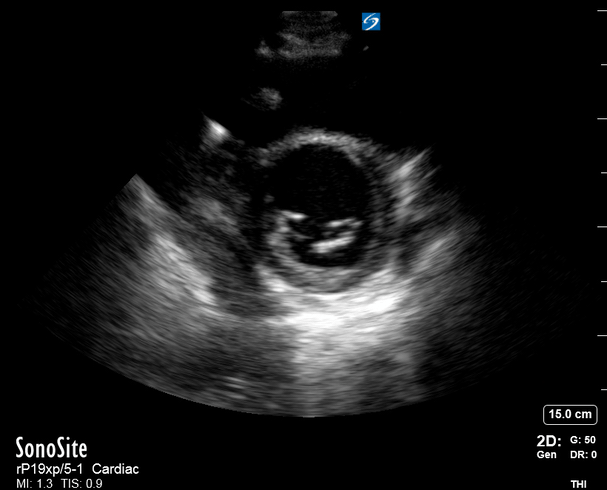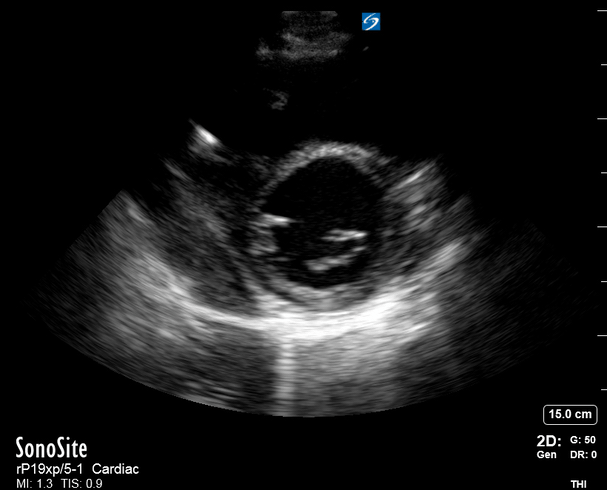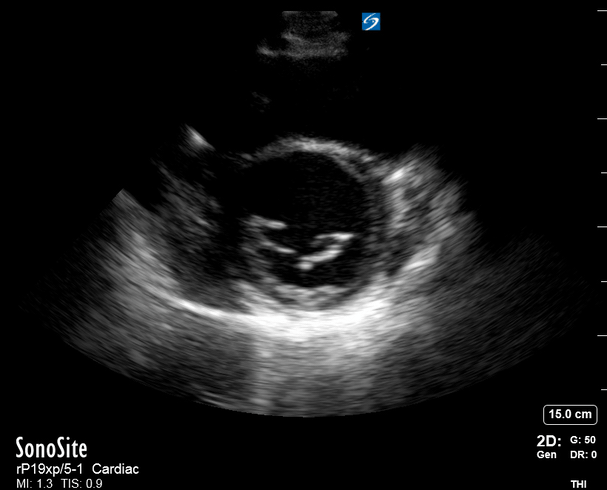
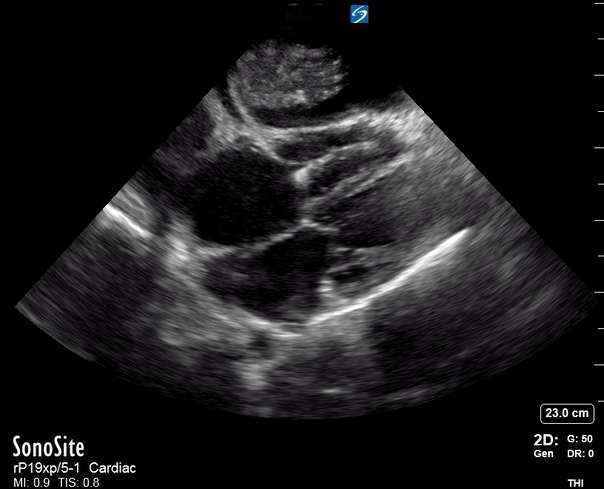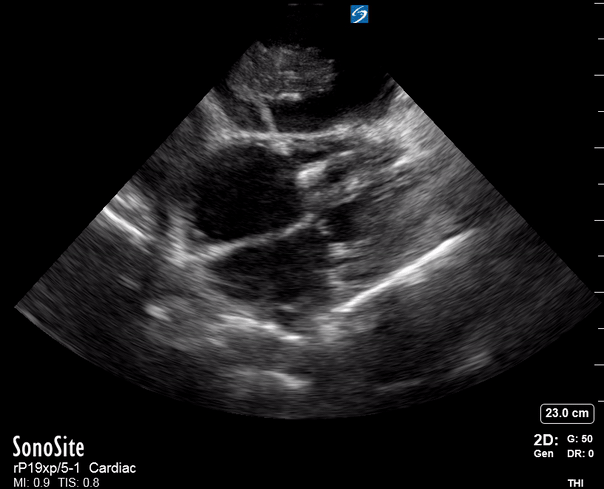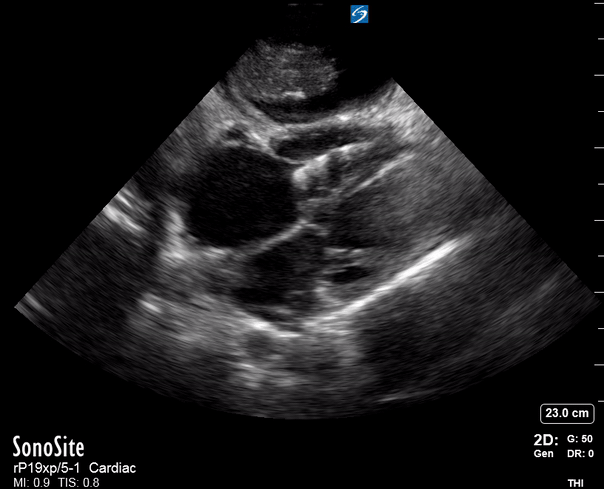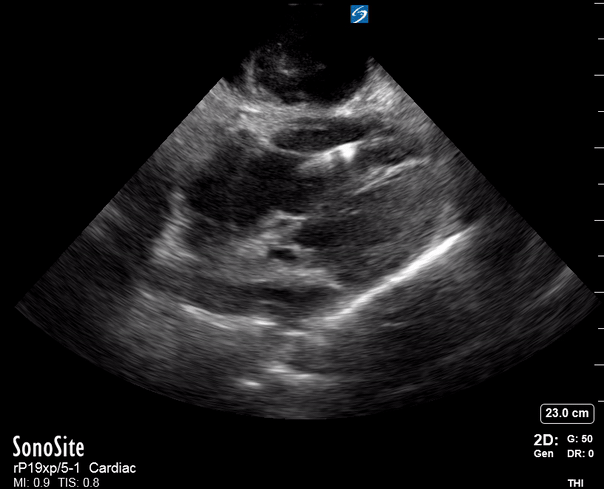
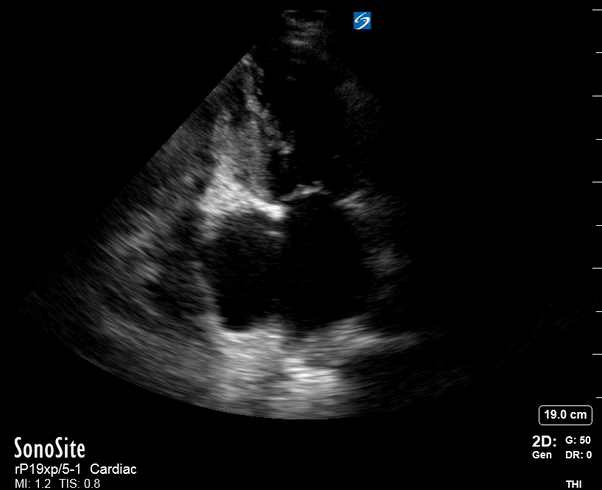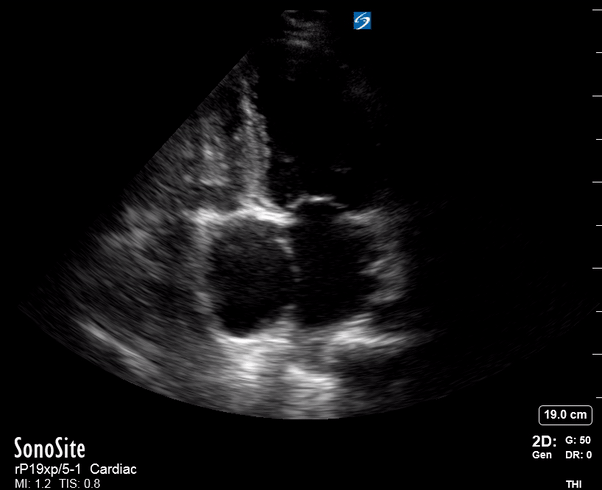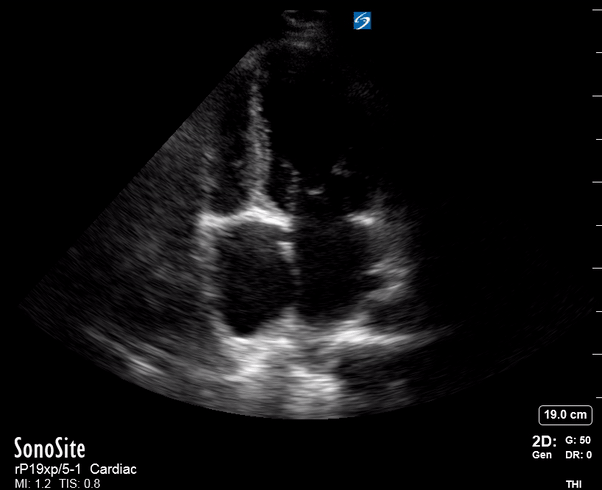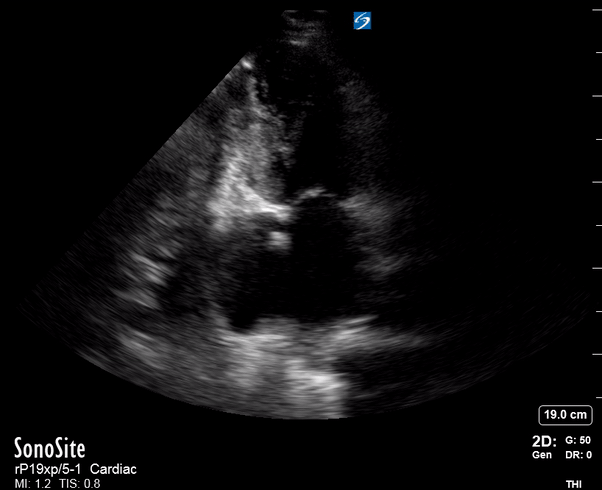
Focused Echo: Interpretation
Rationale.
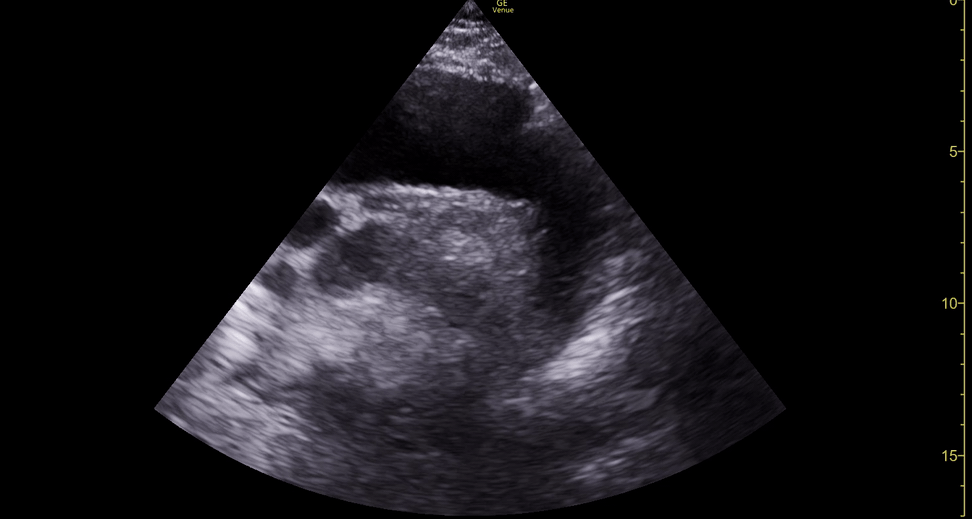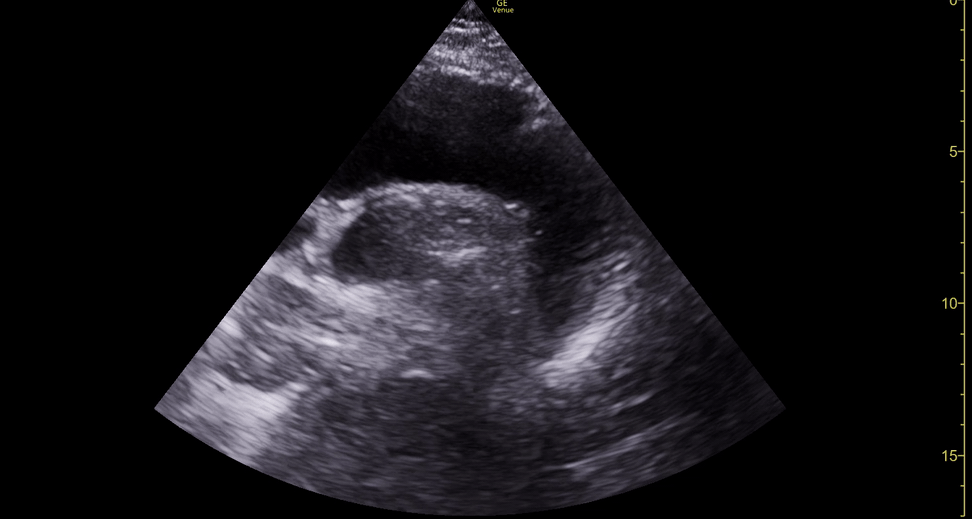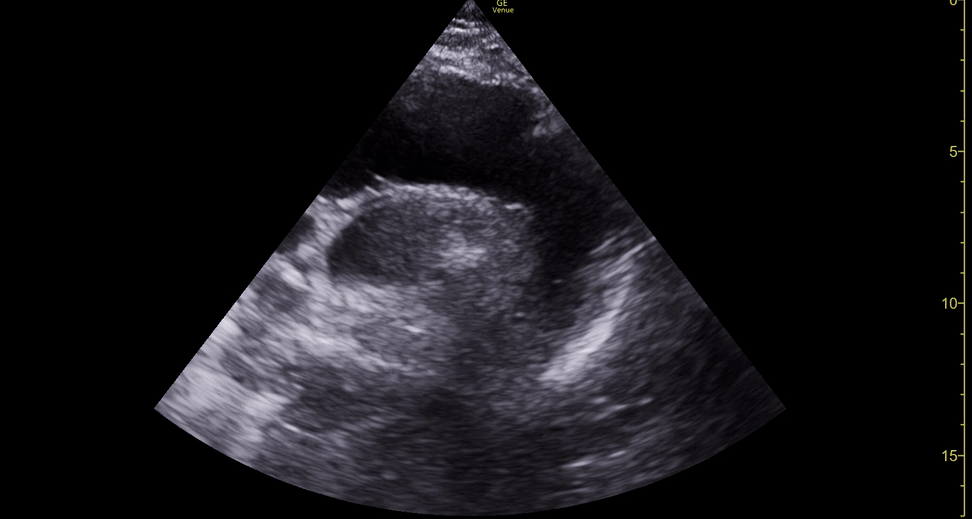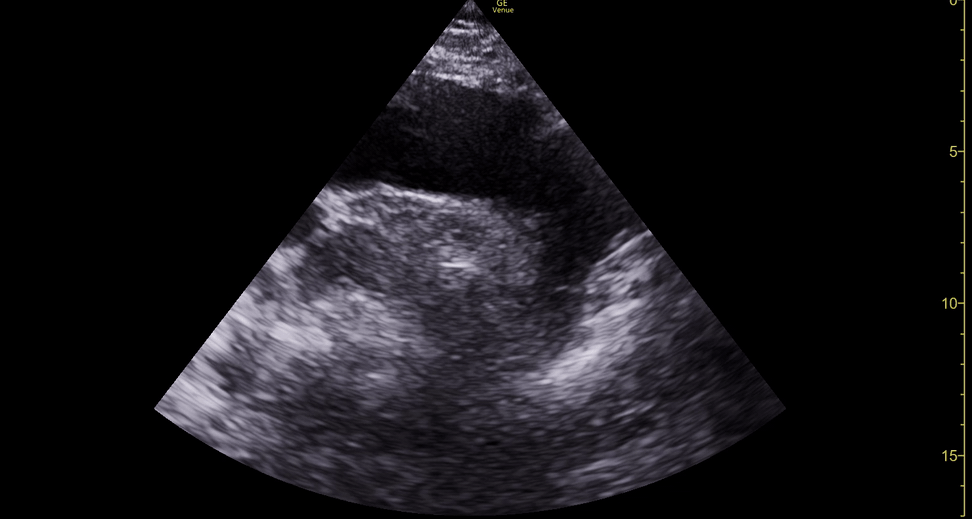
Focused echocardiography is a systematic rapid assessment tool in the acute care setting.
The aim of focused echo is to provide a qualitative assessment of the overall cardiac function by answering specific simple questions (1). It also gives insight into the haemodynamic status, while being safer, faster and easier than other alternatives used with the same purpose, namely invasive central venous pressure (CVP) monitoring (2). Consequently, it’s an invaluable technique to assist the management of chest pain, dyspnoea, shock, and cardiac arrest. When combined with other POCUS modalities (lung, aorta, fast and leg veins) is useful in differentiating shock (3).
THE QUESTIONS?
Focused Intensive Care Echo (1) can help answer the following questions:
Is the LV dilated or significantly impaired?
Is the RV dilated or significantly impaired?
Is there pericardial effusion or tamponade?
Is there pleural effusion?
Is there evidence of low preload?
Are there any other obvious abnormalities?
CLASSIC HAEMODYNAMIC PATTERNS
Haemodynamically unstable patients can present with classic patterns identifiable by echocardiography. These patterns become more obvious and easy to detect as the clinical condition deteriorates:
Poorly contracting LV: Cardiogenic Shock.
Dilated RV, underfilled LV: Obstructive Shock (Pulmonary Embolism).
Pericardial fluid with RV collapse: Obstructive Shock (Cardiac Tamponade).
Vigorously contracting, underfilled LV: Hypovolaemic/Septic shock.
LIMITATIONS: FOCUSED VERSUS COMPREHENSIVE
Formal echocardiography is a comprehensive assessment that involves almost every morphologic and functional aspect of the heart. This skill can only be acquired with extensive formal training under expert supervision, which is outside the practical achievement of most emergency practitioners. When a thorough echocardiogram is required, a formal study should be arranged (4).
The focused approach is simpler and comprises two-dimensional (2D) images without most of the more complex quantitative measurements; hence, it is also easier to master. It has been shown that a rapid goal-directed, focused study can be carried out with a limited amount of training (5). Focused echo has the added advantage that the clinician carrying out the exam is also treating the patient and, thus, is best placed to relate the echo findings to the clinical situation (6).
Even when the general rule is that ultrasound may not delay resuscitation of a shocked patient nor obstruct the management of more pressing issues such as an unstable airway, it should be considered that bedside echo might be fundamental for optimal resuscitation before successful rapid sequence intubation.
Protocol.
Focused Cardiac Ultrasound, as a concept, has various names in the literature, and several academic groups have proposed different protocols (7-10). However, these may include assessments that are too complex for the bedside, not relevant in the acute setting, or incomplete. Furthermore, in emergency medicine, a widespread cardiac protocol (such as FAST for trauma) is still elusive.
FICE & 5E
The Focused Intensive Care Echocardiography (now FUSIC Heart) protocol (1) is sponsored by the British Intensive Care Society and widely accepted within the UK. Although dismissing the aortic root, it provides an adequate overall assessment of the cardiac function based on five simple binary questions:
Is the LV dilated or significantly impaired?
Is the RV dilated or significantly impaired?
Is there pericardial effusion or tamponade?
Is there pleural effusion?
Is there evidence of low preload?
The approach is simple enough to be adopted by Emergency Medicine practitioners, and more importantly, it contemplates a well-structured training process where at least 50 scans are required for sign-off and independent practice.
Alternatively, the 5E Protocol – Entrance, Ejection, Equality, Effusion, Exit (11) – is a standardised and easy-to-remember diagnostic tool with a conceptual framework similar to FICE, making it a good complement in the acute setting. 5E includes a basic assessment of the Aortic root (Exit), and a deeper analysis of preload and IVC (Entrance).
FICE + 5E
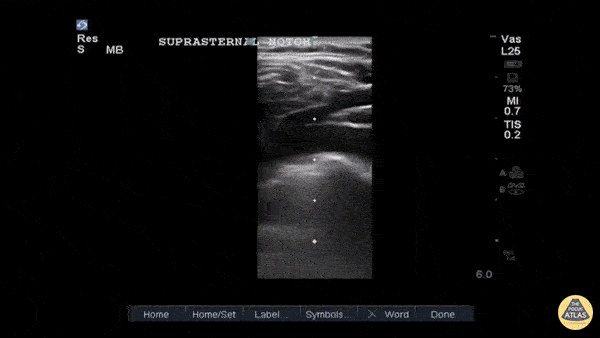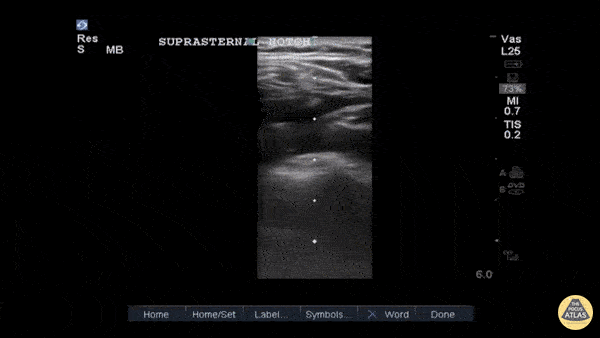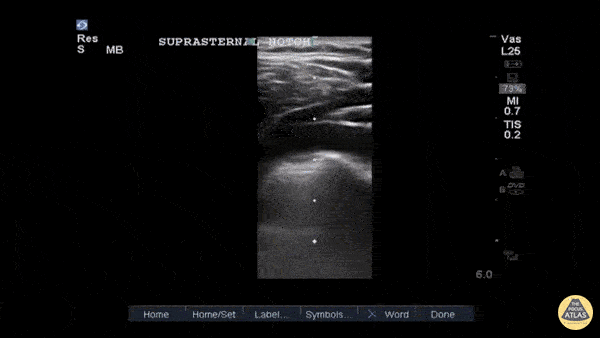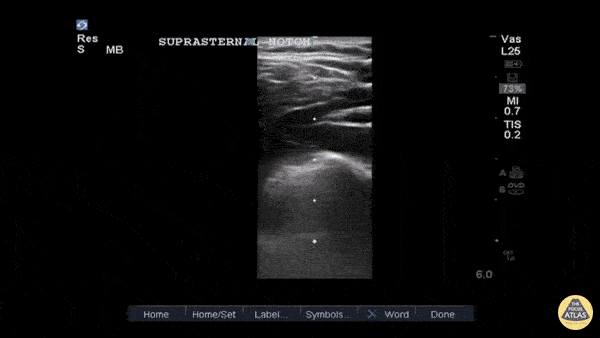
Entrance: is there evidence of low preload?
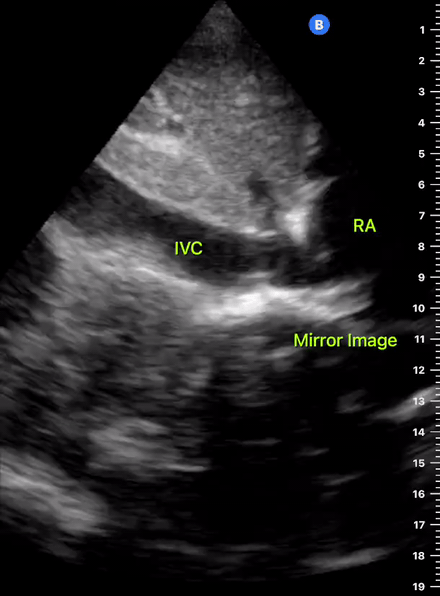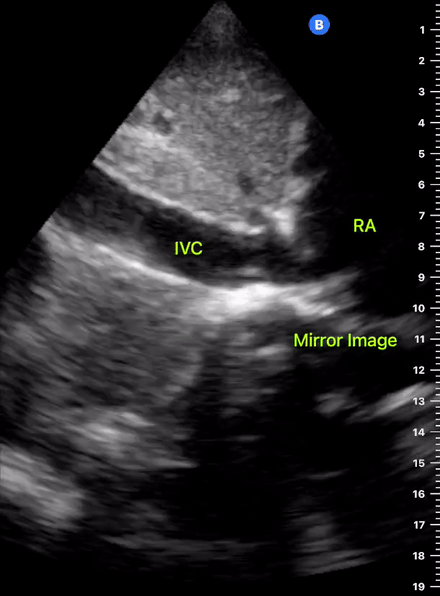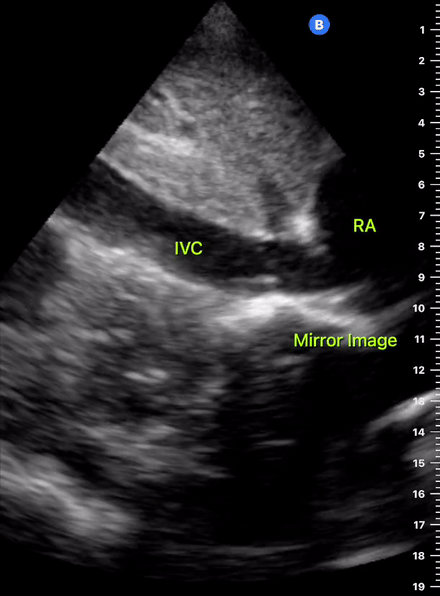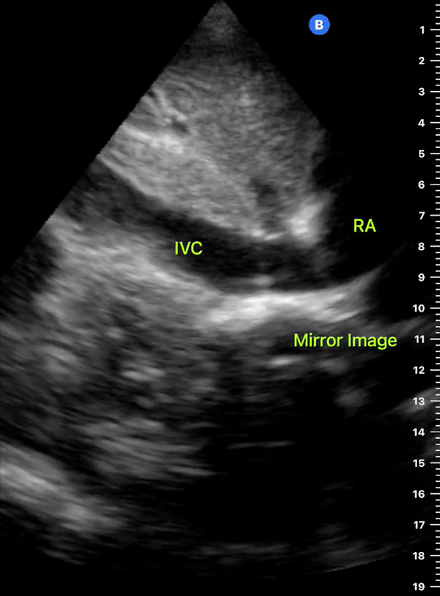
The IVC diameter changes depending on intravascular volume status, and normally, the IVC collapses during spontaneous inspiration. Therefore, the IVC diameter represents a non-invasive method for estimating central venous pressure (CVP). The evidence suggests that in spontaneously breathing patients, there is a good correlation between the sonographic estimation of CVP and values measured with invasive methods (2). IVC filling and CVP, however, allow only a rough correlation with volume status, and the sonographic estimation of preload should at least include the assessment of the LV and the Lung. Thus, it is better to think of IVC filling as an indicator of fluid tolerance, instead of a determinant of fluid responsiveness (6).
IVC exploration is best from the subxiphoid approach with longitudinal and transverse images. The IVC should be assessed in terms of overall size and collapsibility. The diameter is typically measured at its largest (end of expiration) at 1-2 cm distal to where the hepatic veins join the vena cava. An IVC diameter of ≥ 2 cm, especially with minimal or no collapsibility, is considered plethoric and correlates with increased RA pressure. An IVC of < 1 cm, particularly with complete collapse, is considered flat and indicates low preload and potential fluid responsiveness. An IVC diameter between 1 and 2 cm is typically normal.
PITFALLS
In a long-axis view, beware of not sliding off the centre of the vessel, as this will underestimate the size of the IVC and overestimate its collapse. Obtaining long and short axis views may help avoid this pitfall. Another mistake is confusing the descending aorta for the IVC, particularly when scanning in long-axis. Although the IVC may appear to pulsate, the aorta has a thicker wall, and its position is to the patient’s left. Following the IVC upwards will reveal the hepatic veins junction and the entrance to the RA, while the aorta will travel behind the heart. The IVC moves both anterolaterally and craniocaudally with inspiration, and this should be considered during visualization or measuring. For this very reason, measuring in M-mode is not recommended as it would not be accurate.
SHOCK
In a shocked patient, a flat or highly collapsible IVC correlates well with low preload estates (hypovolaemia, haemorrhage, sepsis). Yet by itself, a small IVC is not enough to define low preload and could also represent a normal finding.
Conversely, a distended, not collapsing IVC suggests distal obstruction in a shocked patient. Potential causes include LV failure, massive PE, tension pneumothorax and cardiac tamponade. Nonetheless, there are other causes of elevated cava / RA pressure, such as chronic pulmonary hypertension.
Accurate preload estimation requires clinical context and assessment of cardiac function:
If the IVC is flat, how is the LV managing the preload? Is the LV empty and hyperdynamic? If yes, then there is evidence of low preload.
If the IVC is non-collapsible, how is the RV and the LV managing the preload? Is the LV contractility impaired? is there evidence of obstruction to venous return?
Ejection: is the LV dilated or significantly impaired?
THE VIEWS
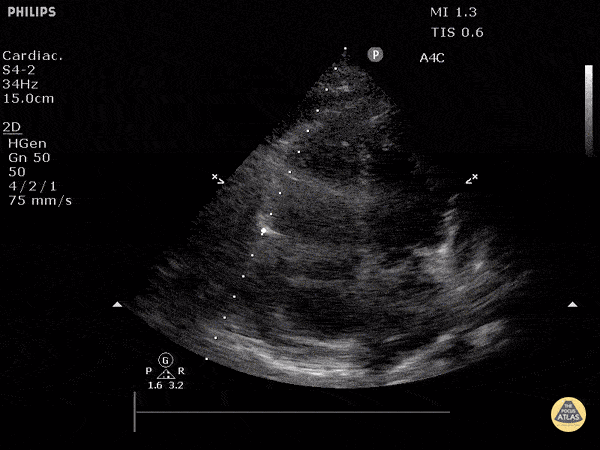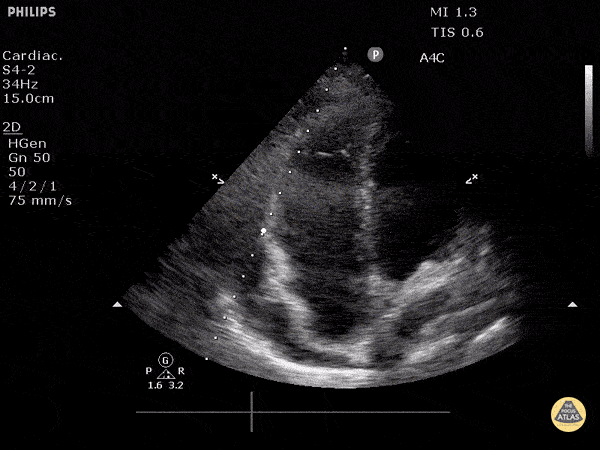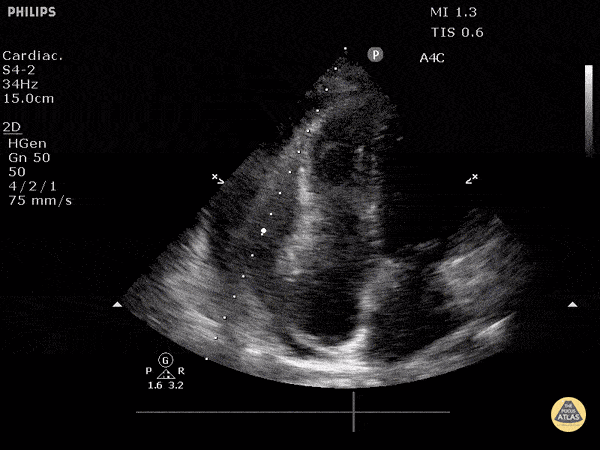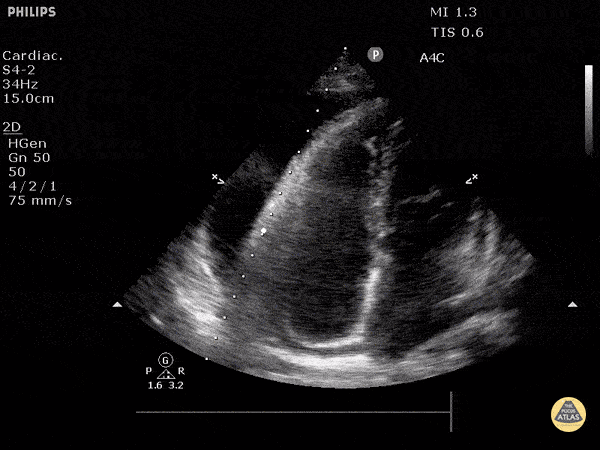
The PLAX view is versatile and allows the recognition of multiple landmarks, making it good for visual estimation of LV contractility. It is essential to optimize the view ensuring a true sagittal long axis, as being oblique to the LV chamber may underestimate its size and overestimate its emptying. The PSAX view at the level of the papillary muscles reveals the entire muscular circumference and concentric squeeze of the LV. It is useful to estimate both global function and focal wall motion abnormalities. The A4C view, although technically challenging, provides good insight into the global myocardial function and chamber size.
LV CONTRACTILITY
Qualitative assessment of the LV and visual estimation of Ejection Fraction is based on three parameters:
Endocardial excursion.
Myocardial thickening.
Movement of the anterior leaflet of the mitral valve.
A qualitative assessment is typically categorised as:
Normal (LVEF 50-65%)
Moderately Depressed (LVEF 30-50%)
Severely Depressed (LVEF < 30%)
Hyperdynamic (LVEF > 65%)
SIGNIFICANTLY IMPAIRED / DILATED LV
A severely depressed LV contractility, particularly when paired with a plethoric IVC or lung B-lines, indicates systolic heart failure. Chronically raised afterload can lead to severe dilation of the LV.
A “non-coordinated myocardial activity” can be recognised during cardiac arrest, and its prognosis is beyond poor.
HYPERDYNAMIC
In contrast, hyperdynamic states are associated with decreased afterload and are classically found in patients with sepsis or severe hypovolaemia. A hyperdynamic heart should be accompanied by a small, collapsing IVC. Moreover, is essential to remember that tachycardic is not the same as hyperdynamic, as the latter is a measure of contractile activity and emptying. A tachycardic heart is not necessarily hyperdynamic.
PITFALLS
While distinguishing normal function from severe dysfunction might be easy, moderate LV depression is more difficult to reliably discern. Also, it should be noted that even with a preserved ejection fraction, heart failure still remains a possible cause of dyspnea. A big percentage of heart failure cases have some component of impaired relaxation, leading to diastolic dysfunction, or heart failure with preserved ejection fraction.
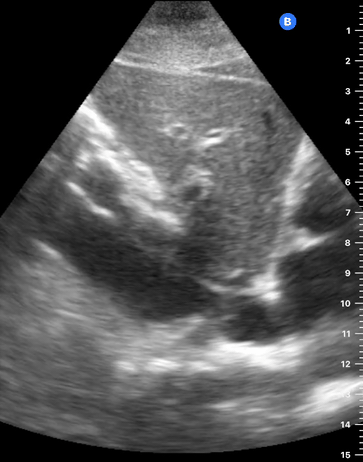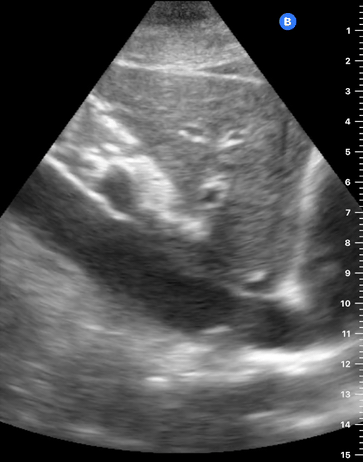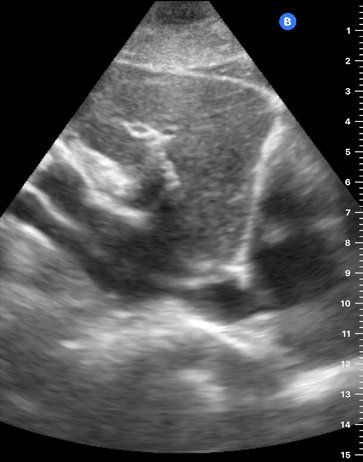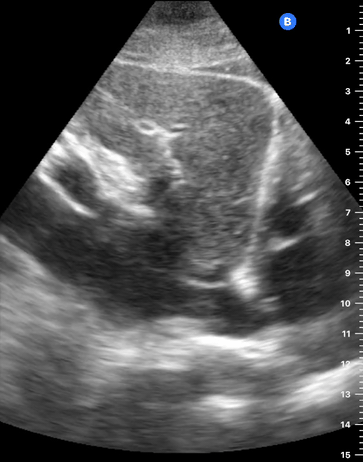
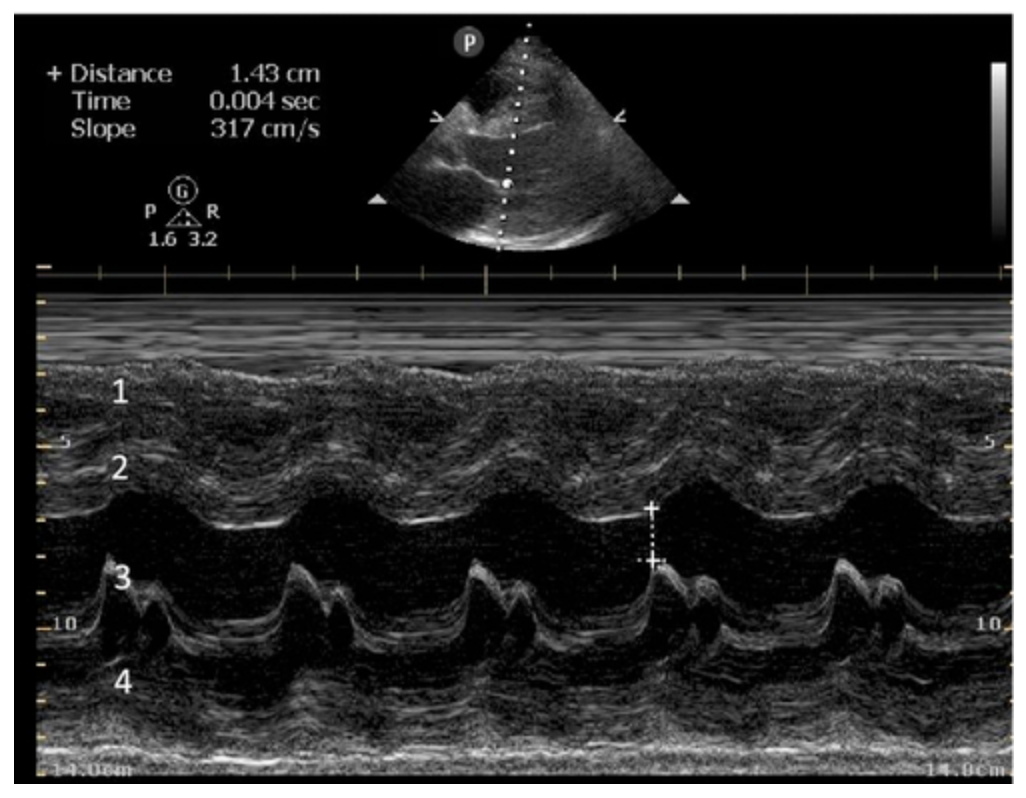
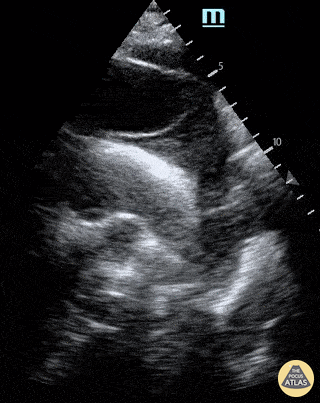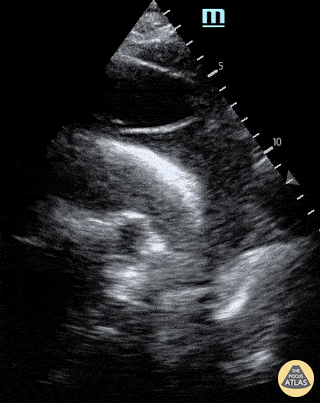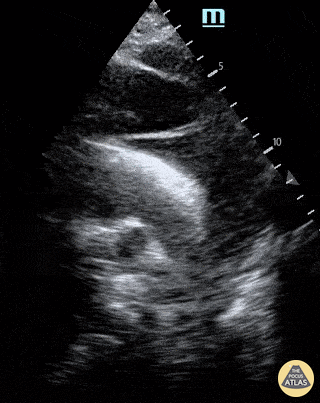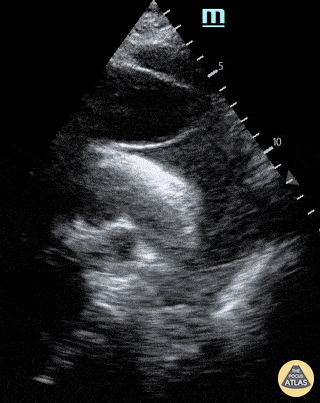
E-POINT SEPTAL SEPARATION
Active movement of the MV anterior leaflet during diastole, so it nearly touches the septum, correlates with good LV filling and ejection fraction. This can be assessed objectively by measuring the E-point septal separation (EPSS), which is the distance between the septum and the mitral anterior leaflet either in B-mode or M-mode. EPSS < 7mm is considered normal. EPSS is a good surrogate measure of ejection fraction, but it should be used with caution, as septal hypertrophy and mitral valve stenosis can lead to wrong estimations. In the PLAX view, it is important to have the septum lying horizontally flat on the image, as an oblique orientation may result in an overestimation of the EPSS.
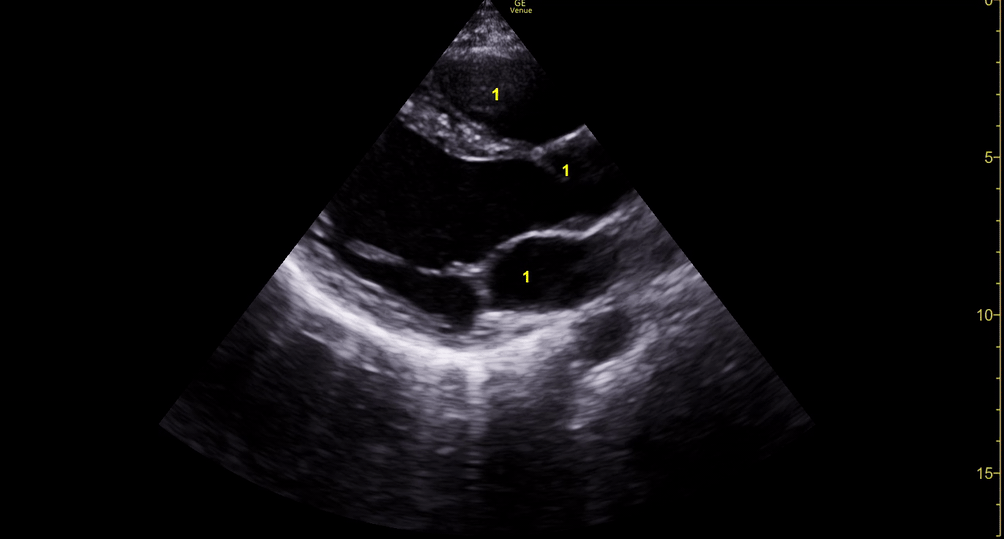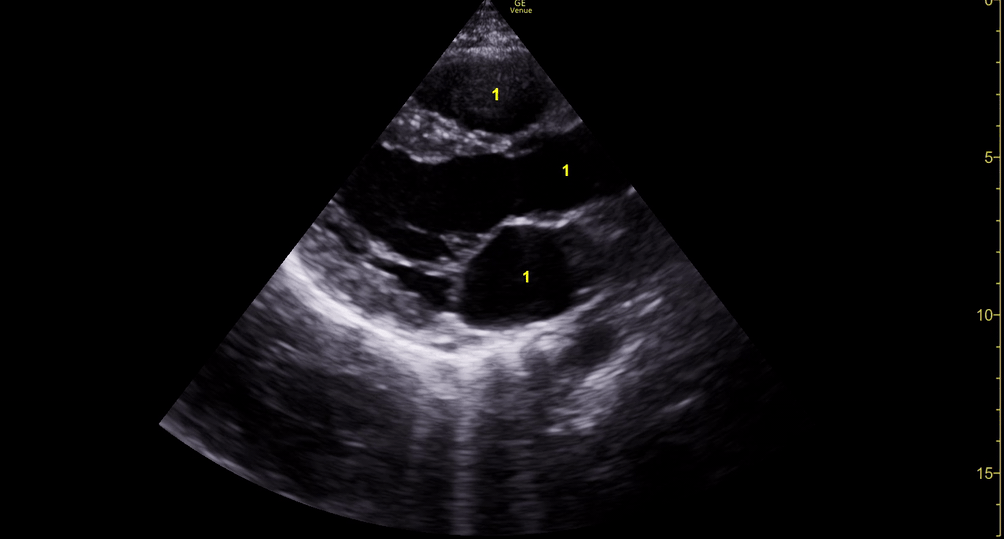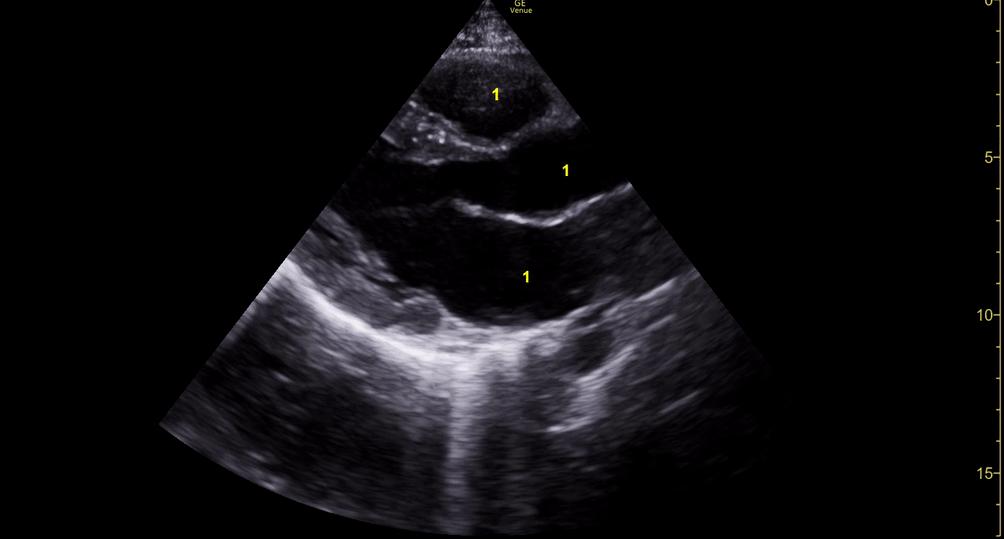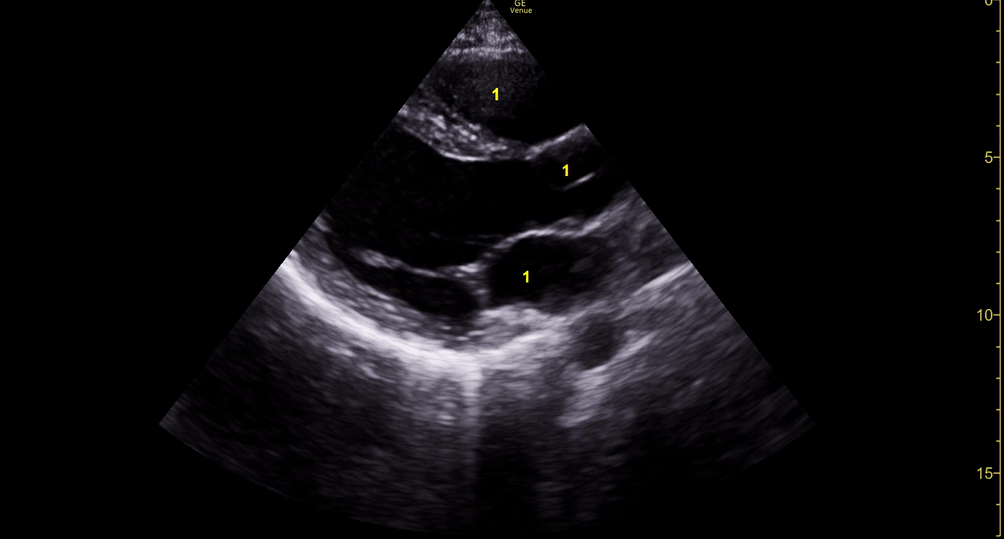
Depiction of E-point septal separation: M-mode is used to measure the distance between the open mitral valve and the ventricular septum. Measurements greater than 7 mm are suggestive of depressed systolic ejection. (1. RV free wall, 2. Interventricular septum, 3. Mitral valve, 4. LV free wall). From: The "5Es" of emergency physician-performed focused cardiac ultrasound (11).
Equality: is the RV dilated or significantly impaired?
Normally, the RV is a low-pressure, thin-walled, high-compliance chamber that wraps anteriorly around the muscular, cone-shaped LV. The normal RV : LV diameter ratio is 0.6 : 1.
When the pulmonary artery pressure rises, the RV will dilate, altering the normal RV:LV ratio. Although sacrificing sensitivity, the use of equality (1:1 ratio) as a cutoff can achieve a specific estimation of RV strain. If imaged correctly by a trained operator, the presence of an RV:LV ratio > 1 is highly specific for RV strain.
RV dilation can be acute, chronic, or acute-on-chronic. However, in patients presenting with undifferentiated chest pain, shortness of breath, hypotension or syncope, the presence of any RV dilation should raise suspicion for acute pulmonary embolism (PE). Furthermore, in a patient in shock, the presence of RV strain may signal the need for aggressive therapy – emergency thrombolysis.
THE VIEWS
The A4C view provides an accurate chamber size comparison. However, achieving a proper A4C view (avoiding foreshortening or ballooning, and visualising the four chambers with a vertically oriented interventricular septum) can be a challenging exercise of image acquisition. Additionally, the PSAX view at the level of the papillary muscles shows both LV and RV side by side and is useful to assess function and size. When RV pressure is high, the septum will be pushed and flattened towards the LV, resulting in the characteristic “D-shaped” LV or “D sign”.
PITFALLS
When comparing size, beware of correct image acquisition, as oblique planes lead to misinterpreting the RV:LV ratio. For apical views be sure to slide the probe sufficiently laterally on the chest wall so that it lies over the true apex. Also, be sure to obtain a real horizontal plane, avoiding foreshortening (ballooning). For the PLAX view it is useful to fan through the heart’s long axis, making sure that LV visualization is maximized relative to the RV. Furthermore, an understanding of probe placement and marker orientation conventions is fundamental. If inadvertently scanning in reverse orientation, the normally larger LV could be mistaken for an abnormally enlarged RV.
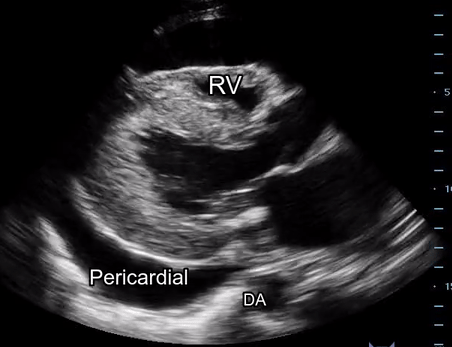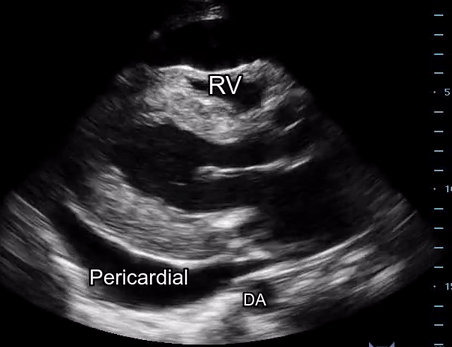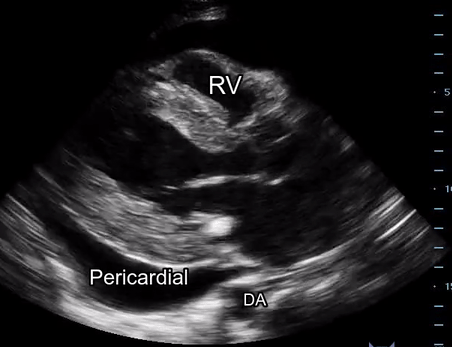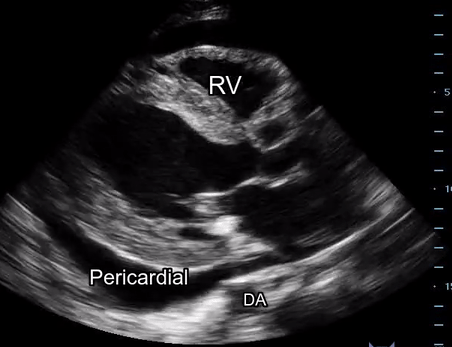
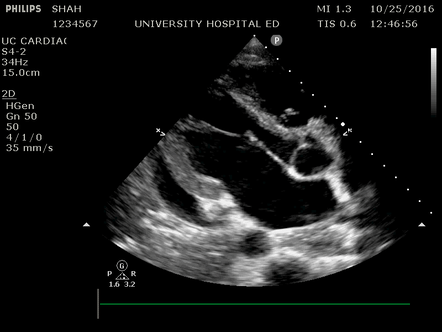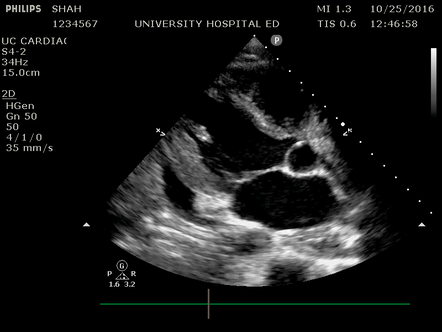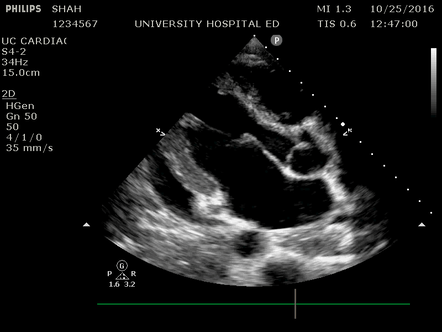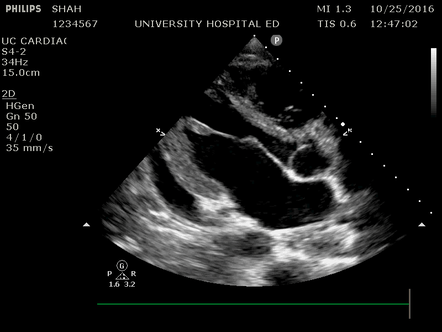
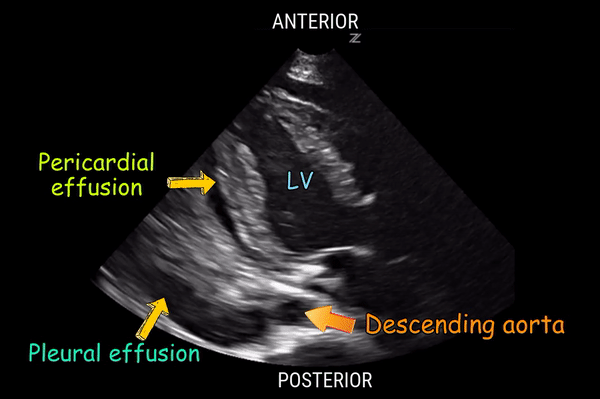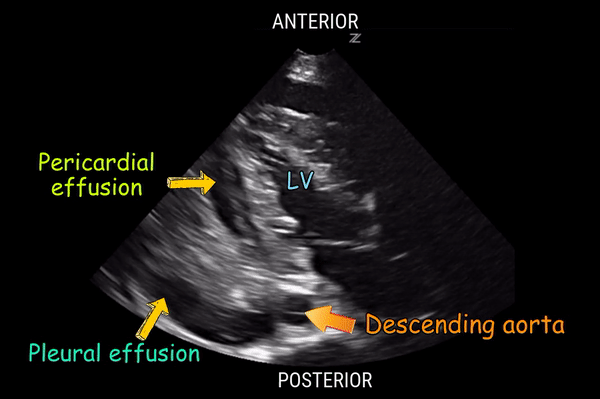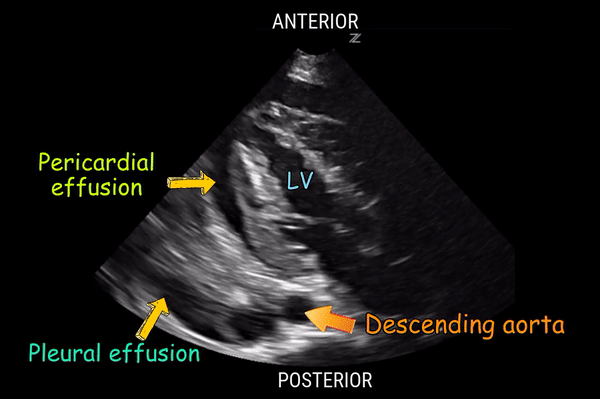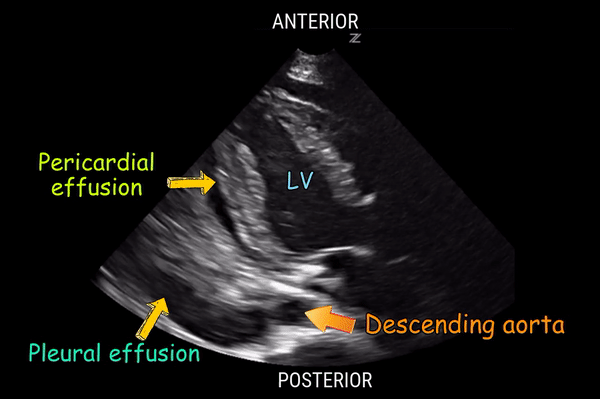
Effusion: is there a pericardial or pleural effusion?
Pericardial effusion is a continuum and can potentially evolve into haemodynamic collapse. Tamponade physiology is detectable earlier with ultrasound than with traditional physical examination, and it has been demonstrated that PoCUS improves mortality in penetrating cardiac trauma. Timely and accurate diagnosis is, therefore, vital.
THE VIEWS
The subxiphoid view is the most reliable for detecting pericardial effusion. Still, it is ideal to obtain different views not to miss a focal effusion as small amounts of fluid can lead to tamponade physiology. Tamponade does not link strictly to the size of the effusion, but correlates more with the speed of onset, causes, and haemodynamic effects.
PITFALLS & PLEURAL EFFUSIONS
A common error is to confuse a pericardial fat pad with effusion. Fatty tissue has a heterogeneous echotexture, moving in coordination with the myocardium, and it cannot be tracked around the heart, especially posteriorly and to the apex. Another pitfall is misinterpreting a pleural effusion as a pericardial effusion. However, they can be differentiated by their relationship to the descending aorta. Pericardial effusion may be seen between the aorta and the LV free wall, whereas pleural effusions are posterior to the descending aorta. Lastly, other causes, namely hypovolaemia and large pleural effusions, can cause RA and RV collapse.
CARDIAC TAMPONADE
Progressively rising pressure translates into evolving tamponade, and this accompanies a series of ultrasound findings:
Initially, the RA collapses during ventricular systole (closed AV valves).
Followed by RV collapse in ventricular diastole (open AV valves).
Ultimately leading to LV collapse.
In addition, the presence of a non-collapsible, plethoric IVC is one of the most sensitive signs of tamponade, and a finding easy to visualise. Conversely, a pericardial effusion in a haemodynamically stable patient with a collapsible IVC is unlikely to represent tamponade.
Chamber collapse can be assessed in M-mode, where the collapsing RV is seen as a notch that takes place right after the mitral valve opening and before its closure (during diastole).
M-mode is used to demonstrate RV collapse (arrow) occurring right after mitral valve opening during diastole. (star = pericardial effusion, 1. RV free wall, 2. Interventricular septum, 3. mitral valve, 4. LV free wall). From: The "5Es" of emergency physician-performed focused cardiac ultrasound (11).
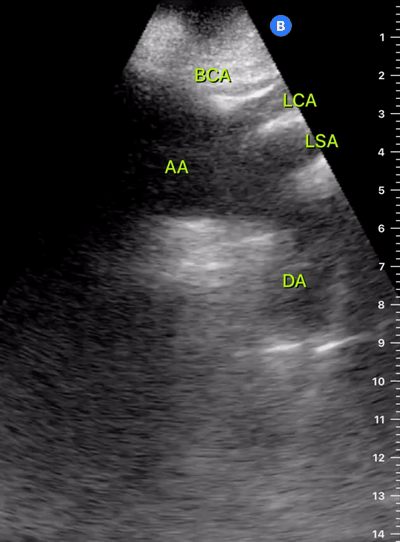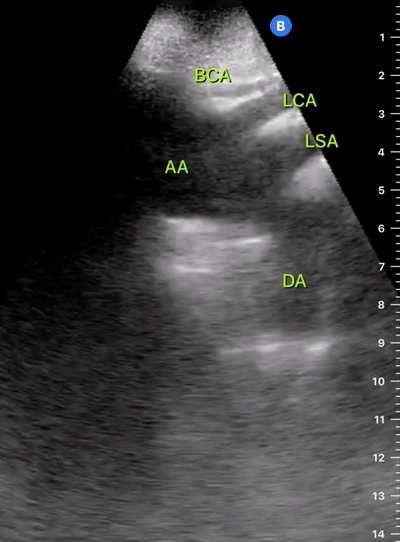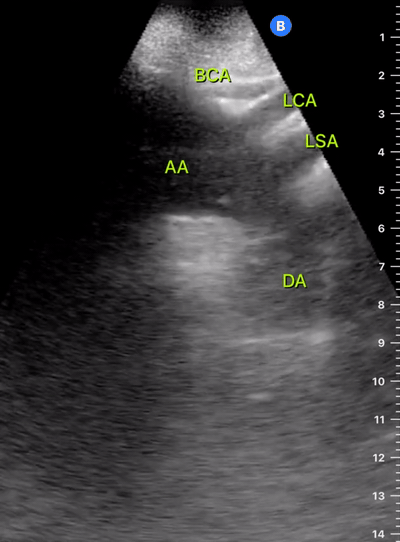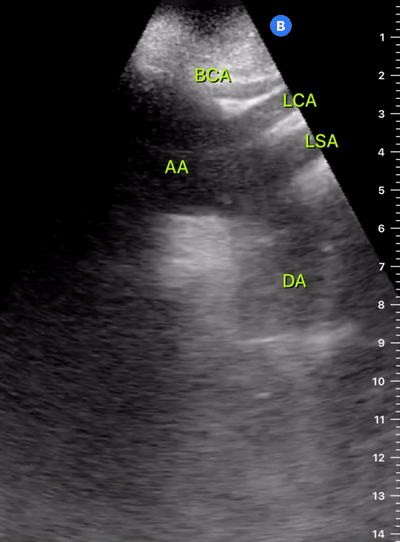
Exit: is there acute aortic root pathology?
Thoracic Aortic dissection is a time-dependent and deadly pathology that can occur silently or be masked by a variety of clinical presentations. While angio-CT remains the gold standard for diagnosis, US may be performed quicker and at the bedside. In the visible portion of the aortic root, the US findings can include intimal flap, aortic valve insufficiency, retrograde aortic flow, or rupture into the pericardium with pericardial effusion and tamponade. Alternatively, dilation of the aortic root is characteristic of a thoracic aortic aneurysm.
Importantly, remember that thoracic aortic dilation or intimal flap may occur distal to the aortic root, where an US scan is usually blind. Hence, the absence of dilation or flap does not rule out aortic disease.
THE VIEWS
The PLAX view is best for exploring the aortic root. If performed by an experienced operator, aortic root measurements in this window correlate well with angio-CT measurements.
The Aortic root size varies with age and gender and should be measured at its widest point, perpendicular to its long axis. In general, a root > 4 cm should be considered borderline and enough to warrant a formal study.
Alternatively, the aortic root size can be estimated by the rule of thirds, where in the PLAx view the size of the RV, AoR, and LA should be roughly 1:1:1.
AORTIC DISSECTION
In both of these views, US may detect an intimal flap seen as a hyperechoic linear structure within the aortic lumen that moves with each heartbeat. The visualization of a flap carries a high specificity and should prompt immediate consultation with cardiothoracic surgery; however, ultrasound sensitivity for intimal flap is significantly low, and its absence does not rule out aortic dissection.
Author: Felipe Urriola-Perez | Review: Pablo Extremera-Navas
Published: 08.12.23
Reference.
FUSIC Heart Training Pathway. Intensive Care Society. https://ics.ac.uk/product/heart.html
Ciozda, W., Kedan, I., Kehl, D.W. et al. The efficacy of sonographic measurement of inferior vena cava diameter as an estimate of central venous pressure. Cardiovasc Ultrasound 14, 33 (2015). PMID: 27542597
Weingart, SD. The RUSH Protocol https://emcrit.org/rush-exam/original-rush-article/
Bowra, Justin; McLaughlin, Russell; Atkinson, Paul; Henry, Jaimie. Emergency Ultrasound Made Easy, Third Edition. 2022. Elsevier Health Sciences.
Randazzo MR, Snoey ER, Levitt MA, Binder K. Accuracy of emergency physician assessment of left ventricular ejection fraction and central venous pressure using echocardiography. Acad Emerg Med. 2003 Sep;10(9):973-7. PMID: 12957982
Basaure, Carlos; Clausdorff, Hans; Riquelme; Felipe. Ultrasonido Clínico, First edition. 2018. Emergency Medicine Residency Program, Universidad Católica de Chile.
Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel RJ. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2013 Jun;26(6):567-81. doi: 10.1016/j.echo.2013.04.001. PMID: 23711341.
https://www.winfocus.org/course/basic-echocardiography/
Aleksandar N Neskovic and others, Focus cardiac ultrasound core curriculum and core syllabus of the European Association of Cardiovascular Imaging, European Heart Journal - Cardiovascular Imaging, Volume 19, Issue 5, May 2018, Pages 475–481, https://doi.org/10.1093/ehjci/jey006
https://www.resus.org.uk/training-courses/adult-life-support/feel-focused-echocardiography-emergency-life-support
Kennedy Hall M, Coffey EC, Herbst M, Liu R, Pare JR, Andrew Taylor R, Thomas S, Moore CL. The "5Es" of emergency physician-performed focused cardiac ultrasound: a protocol for rapid identification of effusion, ejection, equality, exit, and entrance. Acad Emerg Med. 2015 May;22(5):583-93. PMID: 25903585